Team:British Columbia/Project/CRISPR
From 2013.igem.org
iGEM Home



Contents |
CRISPR
What are CRISPRs and how do they work?
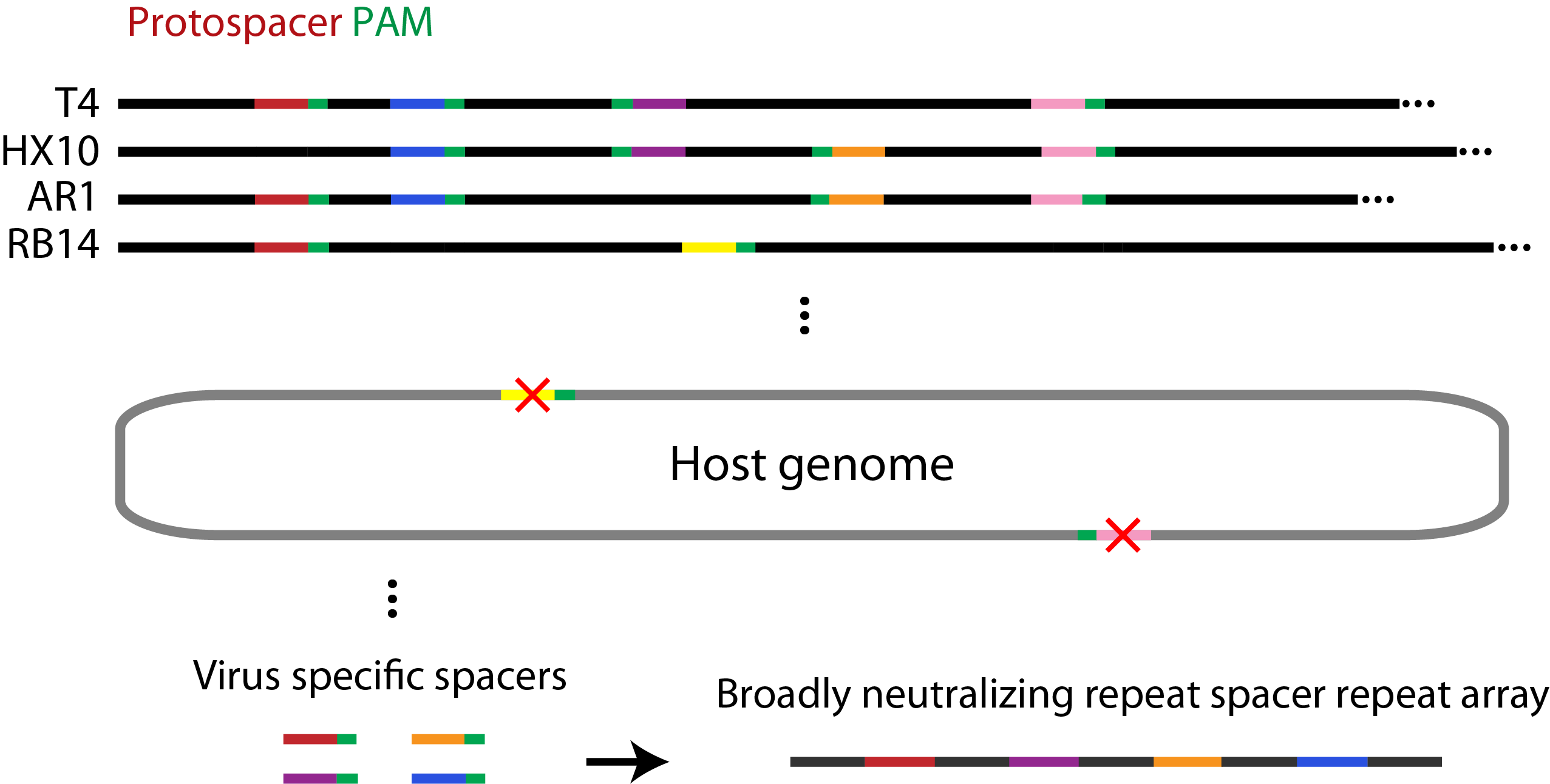
CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats) are specific regions in some bacterial and archaeal genomes that, together with associated Cas (CRISPR-associated) genes, function as an adaptive immune system in prokaryotes. While the details of the ‘adaptive’ part of this immunity are being worked out, it is known that exogenous DNA is processed by Cas proteins into short (~30 base pair) sequences that are adjacent to the Protospacer Adjacent Motif (PAM) site. These short pieces of DNA are then incorporated into the host genome between repeat sequences to form spacer elements. The repeat-spacer-repeat array is constitutively expressed (pre-CRISPR RNAs or pre-crRNAs) and processed by Cas proteins to form small RNAs (crRNAs). The small RNAs are then loaded into Cas proteins and act to guide them to initiate the sequence-specific cleavage of the target sequence.
What did we do with them?
As an adaptive immune response, we wanted to know if the CRISPR system could be put together in a modular way to confer resistance to known phage genomes - vaccinating the host. We first wrote scripts capable of identifying the most broadly neutralizing spacer regions from compiled phage genomes. We then assembled the minimum CRISPR components into BioBricks and conducted the necessary proof-of-concept experiments in E. coli. First, we characterized the dynamics of phage infection in our specific host strain and experimental protocols. We then built a system that protects E.coli against T4 phage infection and are beginning to understand some guidelines for assembling CRISPR components to provide immunity. Currently, we are carrying out further validation experiments, extending our system to work with T7 phage, performing some in vitro characterizations, and exploring new possibilities with our working and the tunable CRISPR system.
Why do this?
Ultimately, we hope that large-scale fermenters could be vaccinated against the collapse caused by environmental phage infection. To extend the application of this approach, we designed specific neutralization elements that allow for population level programming of a culture. We envisioned this first being applied to yogurt where the biosynthesis of flavour combinations might be controlled by targeted phage addition. Check out our population control section for further details on the construction of a few of these pathways. Approaching this from another angle, our modeling section aims to describe the population dynamics of both vulnerable and immunized populations under phage predation with the hopes of eventually being able to rationally tune relative proportions of flavour compounds.
System Design
The three stages of CRISPR immunity (spacer acquisition, crRNA biogenesis and target interferences) and the general gene architecture described above are hallmarks of the CRISPR/Cas system. There is, however, substantial diversity in the naturally occurring spacer elements, the cas genes and the functional PAM sequences. Approximately 45 different gene families have been identified, which, when combined with analysis of their repeat and PAM sequences, can lead to their categorization into three main groups (Types I, II and III) with 10 subtypes (IA, IB, IC, ID, IE, IF, IIA, IIB, IIIA and IIIB).
We chose to work with the type IIA CRISPR3 locus from Streptococcus thermophilus because it has been previously shown to confer immunity to double stranded DNA (dsDNA) phage and plasmids in Escherichia coli and it requires a minimum set of only three components – one protein (Cas9), a non-coding small RNA (trans-activating crRNA, tracrRNA), and the repeat-spacer array. These three necessary components were decoupled from their native regulatory framework and assembled.

Figure 1. SDS-PAGE of Cas9 in 1% arabinose induction.
Cas9
Cas9 is the hallmark of the type II system - a massive 163 kDa multifunctional protein involved in the processing of pre-crRNA and the cleavage of exogenous DNA. The cas9 gene was subcloned out of [http://www.addgene.org/39314/ pMJ824] and assembled under both the arabinose inducible pBAD [http://parts.igem.org/Part:BBa_I13453 BBa_I13453] and constitutive [http://parts.igem.org/Part:BBa_J23118 BBa_J23118] promoters. Cas9 was submitted to the registry as part [http://parts.igem.org/Part:BBa_K1129006 BBa_K1129006]. Expression of Cas9 under both arabinose induction and constitutive expression was confirmed with SDS-PAGE.
Trans-activating crRNA
The tracrRNA is a 36 base, small, non-coding RNA sequence of which 25 bases is complementary to the repeat sequence. The hybridization of the tracrRNA to the repeat sequence in the pre-crRNA results in double-stranded RNA that is recognized and cleaved by a non-Cas RNaseIII enzyme. It has also been shown that tracrRNA, the mature crRNA and Cas9 are all required for cleavage of target dsDNA.
The tracrRNA was cloned from annealed oligonucleotides and assembled under the arabinose inducible pBAD [http://parts.igem.org/Part:BBa_I13453 BBa_I13453] promoter. It was submitted to the registry as parts [http://parts.igem.org/Part:BBa_K1129008 BBa_K1129008] (sense) and [http://parts.igem.org/Part:BBa_K1129009 BBa_K1129009] (anti-sense).
Spacer Selection
Spacers were selected using a perl script (example here) which searches a set of viral genomes to find any occurrences of the PAM site NGGNG. Spacer sequences were removed from the set if (1) the 9 bases preceding the PAM were also found adjacent to a PAM site in the host genome, or (2) if they contained illegal restriction sites. For the remaining spacers, the 30 bases prior to the PAM site were recorded and their frequency of occurrence in each genome was determined.
Figure 2. Summary of the bioinformatics approach used to identify spacer elements broadly neutralizing to several species of phage.
We reasoned the spacers that are the most heavily conserved among related genomes would be under the greatest selective pressure to be retained and would provide a wider range of immunity. Based on this, we selected the spacers which were the most widely distributed in the T7, T4, and closely related genomes for our system. By calculating the fraction of the phage genomes which would be neutralized by combining the most widely distributed spacers, we showed that using our approach only 5 and 3 spacers would be required to neutralize all of the phage related to T4 and T7, respectively.
Toxic Plasmids

Figure 3. Toxic plasmids. CRISPR-mediated cleavage of these plasmids will result in removal of ampicillin resistance, and cause cell death on selective media.
In order to assay for CRISPR-mediated defence against plasmids, we designed two plasmids which, when cleaved, will eliminate the antibiotic resistance conferred by the “invading” plasmid, thereby resulting in cell death. These two plasmids contain the spacer and PAM sequence identified by our informatics pipeline and an ampicillin resistance marker. They have been submitted to the registry (with chloramphenicol resistance) in parts [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1129010 BBa_K1129010] (T4) and [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1129011 BBa_K1129011] (T7).
Results
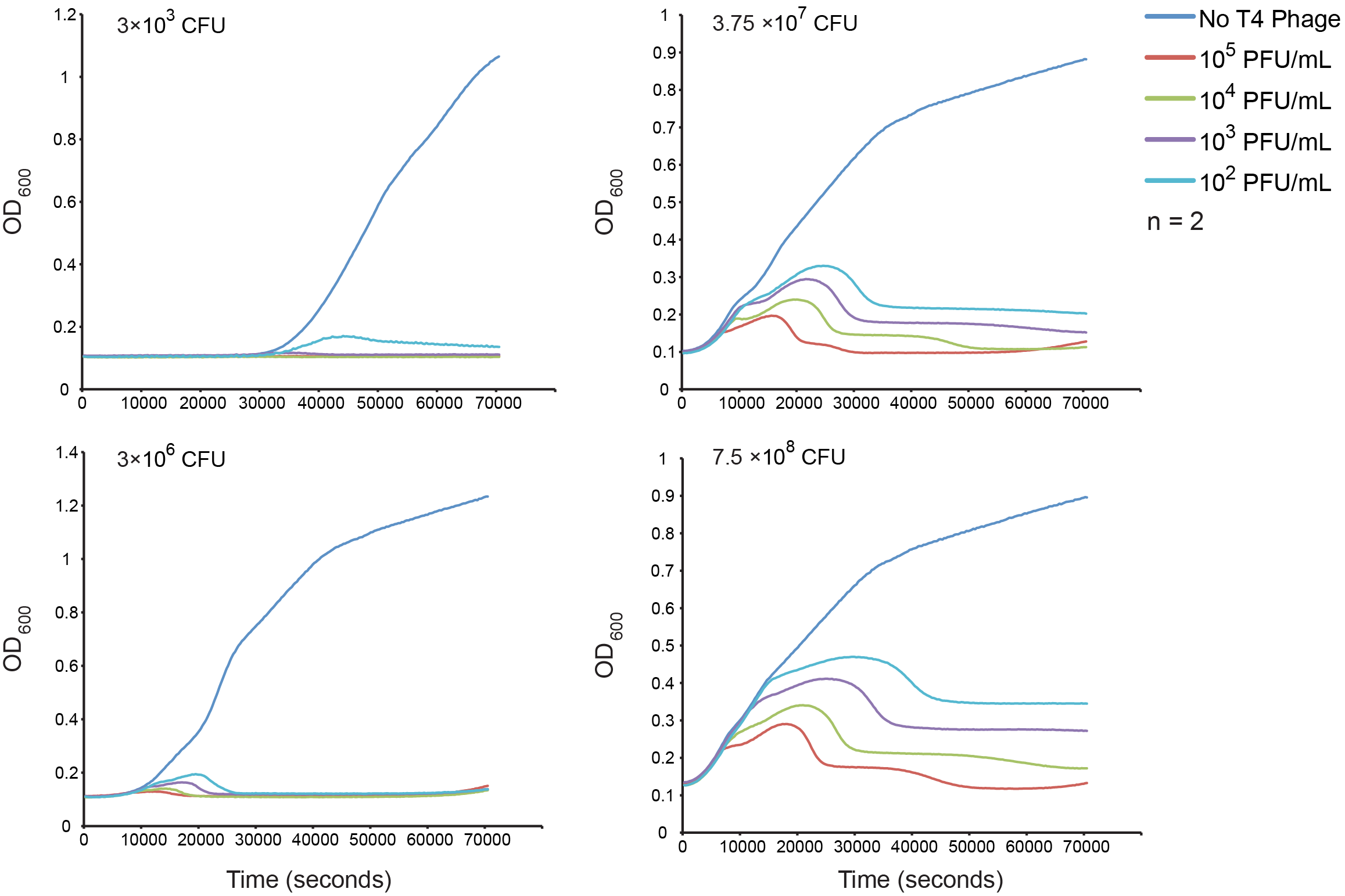
Normal growth under T4 Phage Infection
Our first goal was to measure bacterial growth kinetics under T4 phage predation with our host strain and experimental conditions. The top left corner has the estimated inoculums and four different dilutions of phage were assessed. The results are consistent with a step-wise lysis cycle that is well documented in the literature. See the modeling section for more detailed analysis of the kinetics.
Figure 4. The effect of initial phage concentration on E.coli GB10 cell growth for various inoculums. Overnight cultures of wild-type E. coli 10G cells were grown at 37 °C until an OD of 0.3 was obtained diluted to each inoculum, the cells counts of which were estimate by plate counts (CFU). The cultures were grown in a 96-well format and OD was measured every 5 min for 2 hrs (n = 2).
Combinatorial CRISPR-Cas9 element assembly
Once we were confident in our ability to measure growth under phage predation, we designed a strategy to assemble Cas9, tracrRNA (sense/antisense) and the T4 spacer-repeat-spacer with inducible promoters in a combinatorial pattern and made a library of approximately 800 clones (see protocols). We then added 103 phage to each clone and screened for growth in high-throughput.
Figure 5. Measuring OD of combinatorial library clones after incubation for 24 hours at 30 °C.The horizontal red line represents the threshold for selection of clones for sequencing and colored points correspond to characterization data in the subsequent figure. 103 phage were added to each well.
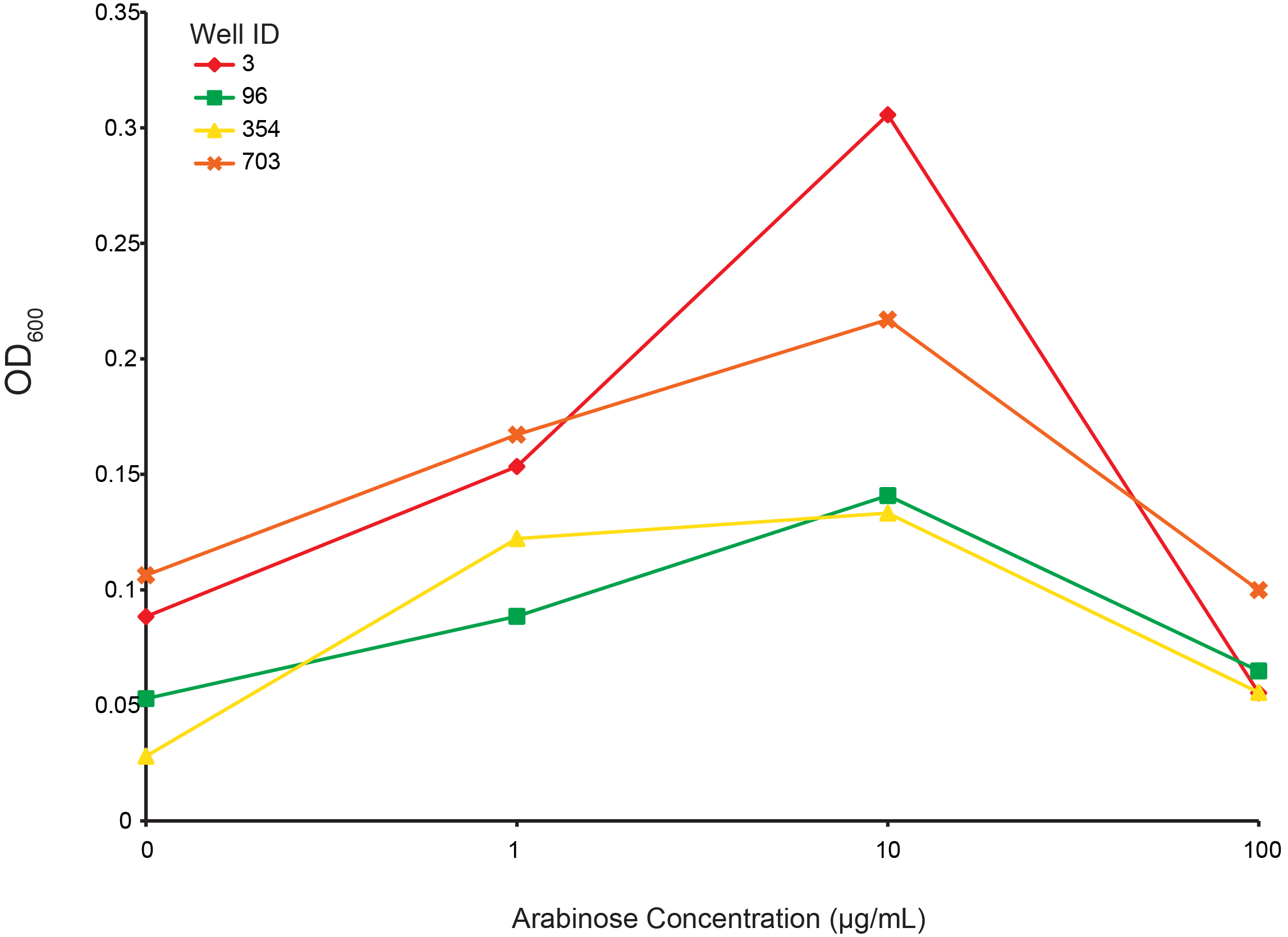
Arabinose dependent growth under T4 infection
From the preceding experiment, we selected four clones for characterization (highlighted in figure 2; red, green, yellow, orange are wells 3, 96, 354, 703, respectively). As all the CRISPR components were on arabinose inducible promoters, we looked for the arabinose dependant survival of selected clones under phage predation. An intermediate level of induction gave the largest final OD and suggests that high induction of Cas9 or other components creates burdens on the cell. These data also supports the CRISPR construct being responsible for escaping phage predation.
Figure 6. Measuring final OD of select combinatorial library clones under different arabinose concentrations after incubation for 24 hours at 30 °C. Well IDs correspond with the precending figure. 103 phage were added to each well.
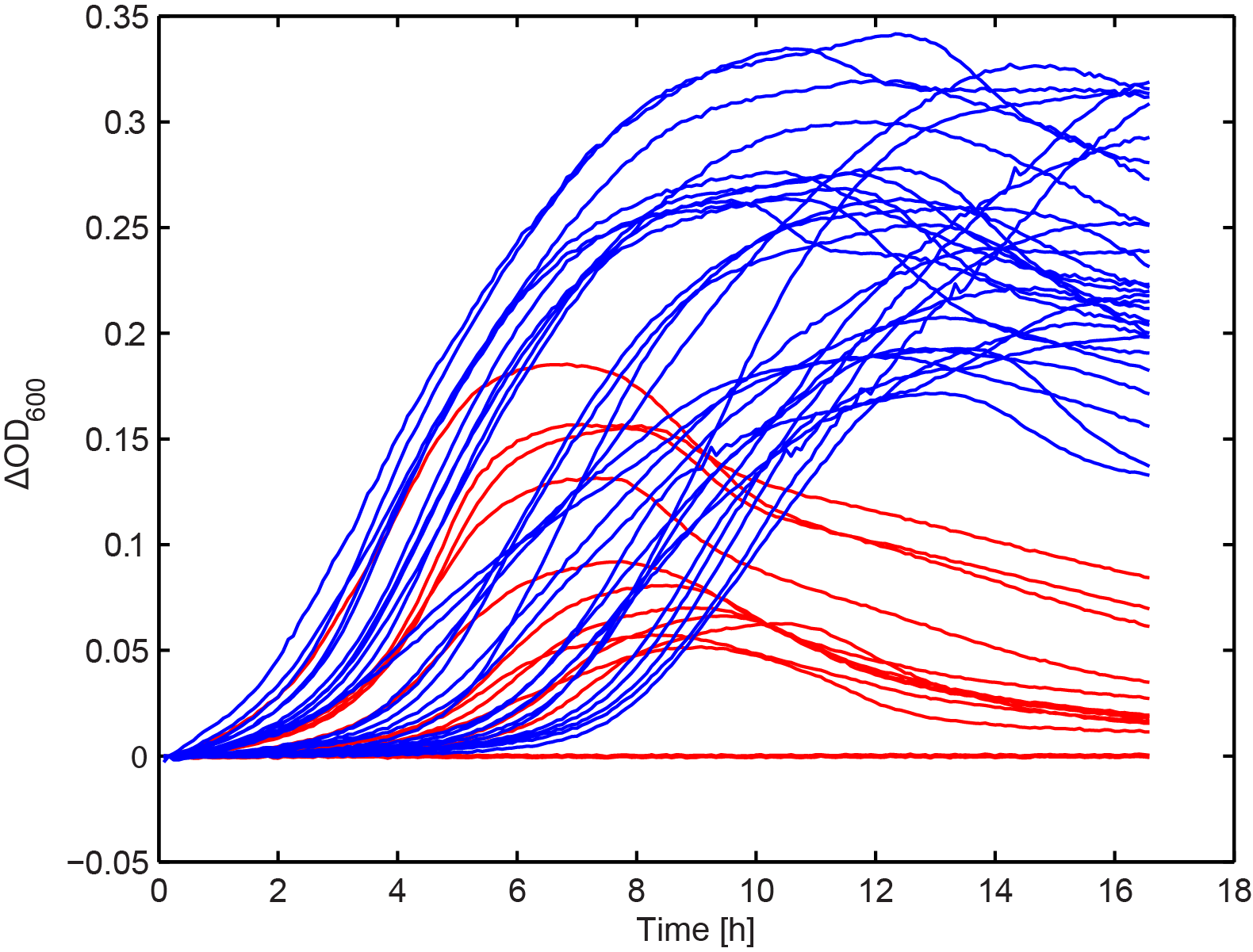
Growth kinetics of immunized culture
From the combinatorial library, we performed colony PCR on 30 clones and showed that the majority of constructs had an approximately 5 kb long insert. A subset of these, along with the clones selected in Figure 2 were arrayed into a 96-well plate. We then added 103 T4 phage to each well and measured OD in the same way as in Figure 1. This seemed to show that there were two distinct groups, one with increased fitness in the presence of phage.
Figure 7. The effect of phage predation on clones from combinatorial libraries. Clones were arrayed into a 96-well plate and grown for 6 hours at 30 °C. 103 phage were then added to each well and OD was monitored overnight. The results seem to resolve two groups, one showing increased final OD over the other (blue and red lines respectively (n=2).
Finally, we added a inoculum of cells that corresponded our initial characterization of phage predation (Figure 1) and monitored growth of two select clones from the two different groups identified in the preceding experiment (Figure 4). This showed an interesting dip in growth at approximately 10,000 seconds, which we are in the process of characterizing. Also, we have yet to confirm the exact orientation and composition of the clones from the combinatorial library. Once this is done, however, we are expecting to be able to comment on the minimum set of CRISPR components required for resistance and on any difference with sense vs. antisense tracrRNA.
Figure 8. Monitoring growth of two select combinatorial library clones under high cell inoculum. 10^3 phage were then added to each well and OD was monitored overnight (n=2).
 "
"