Team:Paris Saclay/Notebook/August/12
From 2013.igem.org
Contents |
Notebook : August 12
Lab work
A - Aerobic/Anaerobic regulation system
Obtaining Pfnr_RBS-LacZ-Term in PSB1C3
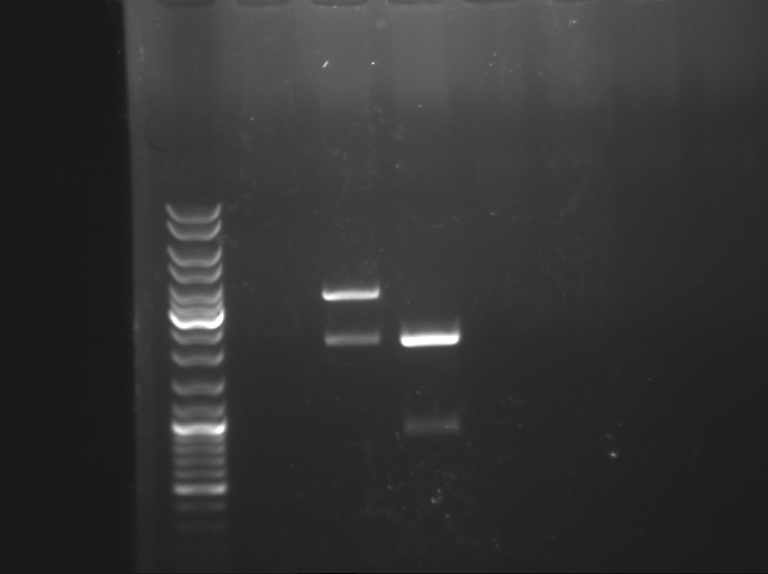
1 - Digestion of Bba_K1155000, Bba_K1155007 and Bba_K1155003
Anaïs, Nadia, XiaoJing
Protocol : digestion
Used quantities :
- Pfnr in PSB1C3 (Bba_K1155000) :
- Buffer : 5µL
- H2O : 38µL
- Pfnr : 5µL
- SpeI : 1µL
- PstI : 1µL
- RBS-LacZ-Term in PSB1C3 (Bba_K1155007) :
- Buffer : 5µL
- H2O : 23µL
- RBS-LacZ-Term : 20µL
- XBal : 1µL
- PstI : 1µL
- RBS-AmilCP-Term in PSB1C3 (Bba_K1155003) :
- Buffer : 5µL
- H2O : 33µL
- RBS-AmilCP-Term : 10µL
- XBal : 1µL
- PstI : 1µL
Expected sizes :
- RBS_LacZ_Term : 3500 kb
- RBS_AmilCP_Term :
- PSB1C3 :
2 - ObtaµLining RBS_lacZ_ter.
Abdou
Clone 10,14 and 15 plamid extraction using nucleospin kit.
Protocol : hight copy plamid extraction
Results: concentration measured by nanodrop
- clone 10: C=38ng/µl 260/280= 1.78
- clone 14: C=48.5ng/µl 260/280=1.90
- clone 15: C=52 ng/µl 260/280=1.78
We still have to sequence plasmids in order to verify our results.
A - Aerobic/Anaerobic regulation system / B - PCB sensing system
1 - Gibson assembly.
August 1st PCR purification to be sure about the experiment. BphR2 part1, BphR2 part2 and RBS_BphR2_part1, FNR part1, FNR part2 and RBS_FNR_part1.
Protocol : PCR_clean_up
Results:
| IMAGE |
|
we have no fragment so we must do again these PCRs
 "
"