Team:TU-Munich/Project/Localisation
From 2013.igem.org
Localization in Physcomitrella patens
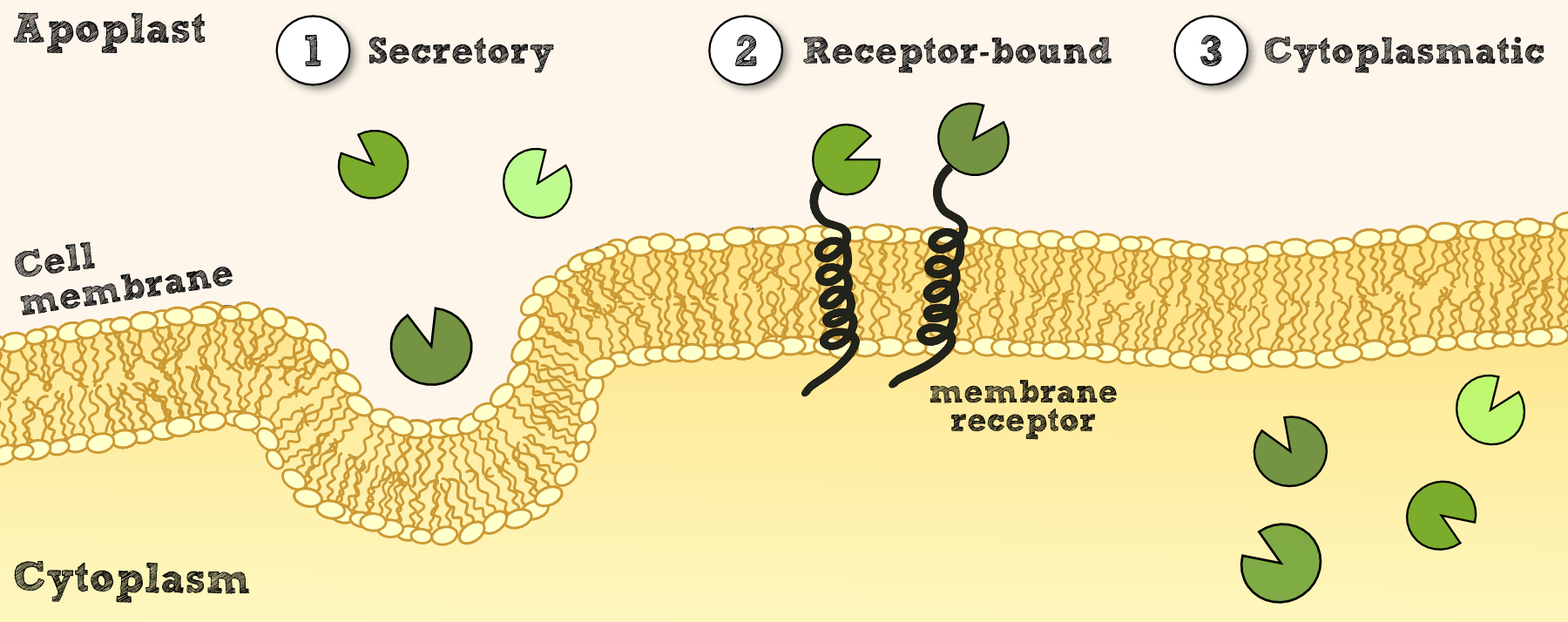
In order to use Physcomitrella as a chassis for Phytoremediaton, it is essential to be able to express effector proteins in different compartments. This includes cytoplasmatic expression of cytosolic effectors which degrade xenobiotics capable of crossing the cell membrane and which might depend on cofactors for degradation or conjugation. Secondly, there is the possibility to secrete effectors outside of the cell for an easy access to their respective target molecules. Other applications benefit of the expression of immobilized effectors on the inner or outer cellular membrane. This allowing the creation of systems that dont release transgenic proteins into the environment and of systems able to internalize substances attached by recombinant binding proteins.
Cytosolic expression of effector proteins is achieved by cloning the respective BioBrick downstream of the Actin_5 promoter in either RFC 10 or RFC 25. The secretion of effectors is achieved through the addition of signal peptides of an antibody [Fussenegger] and also tested the signal peptide from the SERK receptor of Physcomitrella patens which both should be suitable BioBricks to accomplish secretion. For this purpose the signal peptides were created as RFC 25 N-parts and the effectors need to be availible in RFC 25 to create fusion proteins. Finally the inclusion of recombinant effector proteins into a receptor which is functional in Physcomitrella patens was investigated by the construction of a synthetic receptor based on the SERK receptor
Cytoplasmatic Expression
Cytoplasmatic expression of effector proteins is necessary, when the effector is either dependent on cofactors that only exist in the intracellular milieu or when it's mechanism is connected to at least one intracellular component which does not appear outside of the cell. For our project, this is the case for:
- Catechol-1,2-dioxygenase: needs ferredoxins to regenerate the oxygen-inactivated iron
- Glutathion-S-transferase: needs glutathion for coupling reactions
Secretory Expression
When some effectors proteins contain disulfid bonds, that effector has to be secreted as the cytosolic compartment does not allow formation of disulfid bonds due to its reducing milieu. For secretion, that protein has be fused to a signal peptide for translocation into the endoplasmatic reticulum. For this purpose we created two biobricks that code for a signal sequence for endoplasmatic reticular translocation, namely SERK signal peptide ([http://parts.igem.org/Part:BBa_K1159303 BBa_K1159303]) of the Somatic Embryogenesis Receptor Kinase from Physcomitrella patens and the IgKappa signal peptide of the Ig Kappa chain from Mus musculus ([http://parts.igem.org/Part:BBa_K1159304 BBa_K1159304]). In our case, following effector should be secreted:
- Laccase from Bacillus pumilus: has 1 disulfid bond
Membrane-bound Expression
For substance binding proteins or as a alternative to secretion for degrading enzymes, it would be convenient to anchor the effectors on the surface of the plant, namely the cytoplasmatic membrane.
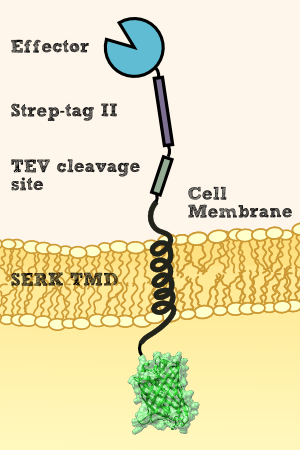
For this purpose, we did not olny add a signal for secretion at the N-terminus of our effector protein, but also have to added a downstream transmembrane domain from Physcomitrella patens ([http://parts.igem.org/Part:BBa_K1159315 BBa_K1159315]) for membrane localization. Furthermore, we fused GFP ([http://parts.igem.org/Part:BBa_K1159311]) to the intracellular site of the transmembrane region in order to be able to verify membrane localization via fluorescence microscopy. Between the effector protein and the transmembrane region, we added a linker containing Strep-tag II and a TEV cleavage site. This allows us to characterize the membrane-bound effectors by incubating the plant with TEV Protease, which causes the release of membrane bound effectors. Hence, purification via the Strep-tag II and successive characterization of the effector is possible.
For more details on the design process of the transmembrane region, see Protein Predictions.
Modularisation: Employing SpyTag & SpyCatcher for post-translational fusion
To create transgenic moss able to degrade customized combinations of xenobiotics targeted by different effector molecules, we introduced the SpyCatcher/SpyTag system into our project http://www.pnas.org/content/109/12/E690 Bijan Zakeri et al., 2012. This system was created for posttranslational protein fusion based on a covalent bond which is formed between the side chains of residues of SypCatcher and SpyTag.
Recombinant effector proteins fused to the SpyTag can thus be expressed separately (e.g. at a different intensity, via a secretory pathway) from receptors fused to the SypCatcher. It enables us to express the SERK-receptor controlled by a strong promoter and to individually adjust the expression of different effector proteins according to their respectively desired concentration. (Fig. 3.2)
Another application lies in the fusion of multimeric effector proteins to receptors, where the fusion of all individual subunits to the receptor is not a viable option due to steric reasons. The SpyCatcher/SpyTag system bypasses this problem as it allows the multimeric protein to assemble into its active form before its immobilization on the exterior membrane by a respective receptor. (Fig. 3.3)
References:
- http://www.plantphysiol.org/content/127/4/1430 Schaefer and Zryd, 2001 Schaefer, D.G. and Zrÿd, J. (2001). The Moss Physcomitrella patens, Now and Then. Plant Physiology, 127(4):1430-1438.
- http://www.ncbi.nlm.nih.gov/pubmed/22366317 Zakeri et al., 2012 Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M. (2012). Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci U S A. 20;109(12)
 "
"




AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351