Team:Leeds/Results
From 2013.igem.org
Results from the lab so far. Please note, many of the images have been uploaded at high resolution, and are best viewed from their file page. Access this by clicking the image. We may later introduce a gallery of the images to maximise the screen usage, but this is dependent upon Coffee-Induced coding by the Code Monkey.
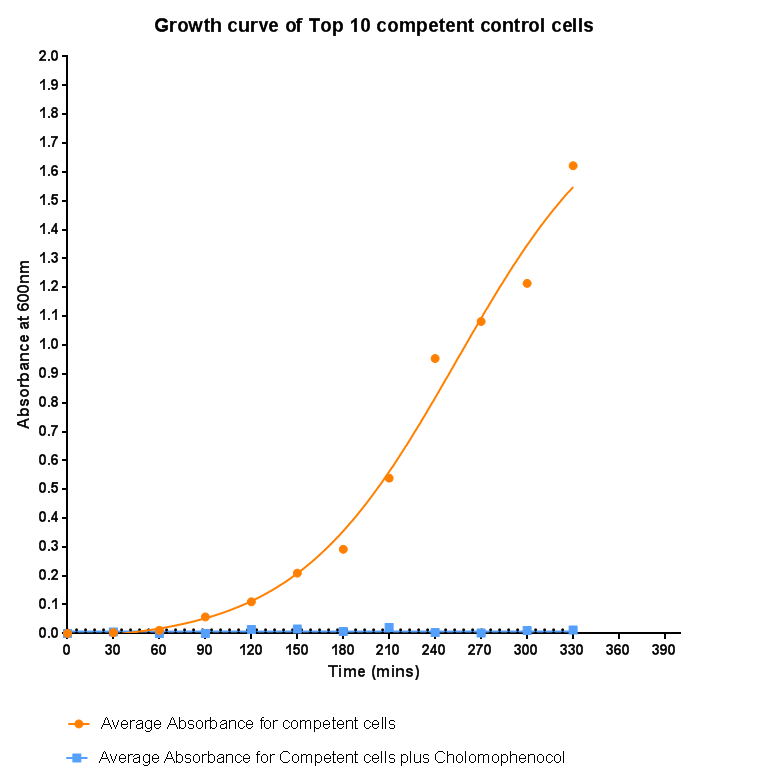
Bacterial growth curvesWe are monitoring the growth of our bacteria containing our devices to see if the genes we inserted have any effect on bacterial growth. Control Bacterial Growth CurveIn this experiment we used our chosen expression cells and monitored their growth on SB media both in the presence of chloramphenicol and without chloramphenicol. As the bacteria do not contain our plasmid that codes for chloramphenicol resistance, we didn't expect them to grow when it was present. The graph produced from the absorbance measurements taken over time, shows the growth of the bacteria. It has a clear lag and log phase when the bacteria are grown in SB media only. But as expected, the graph shows that the bacteria do not grow in the presence of chloramphenicol. This graph will be used as a control and other growth curves of bacteria containing our device will be compared to it in order to determine whether the genes we inserted cause an effect on cell growth.
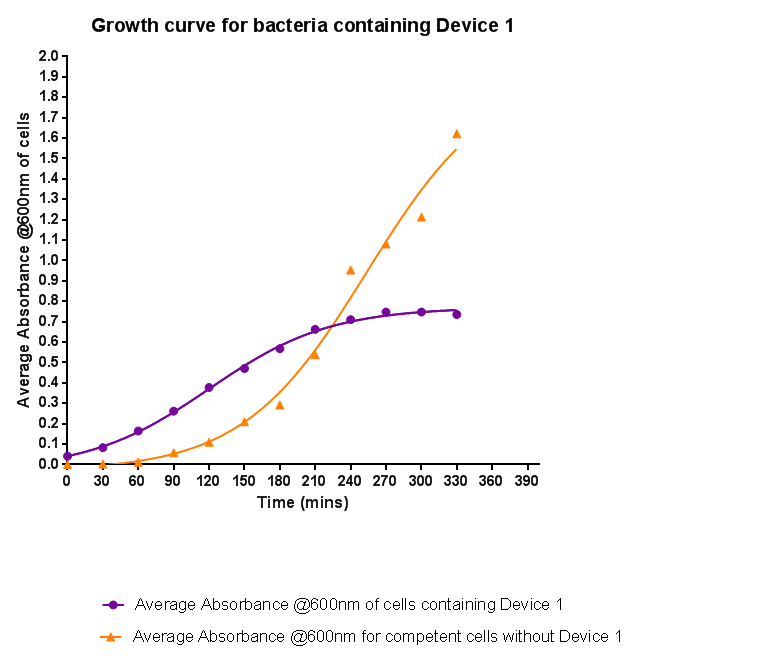
Characterisation of Device 1Growth CurveBacteria were grown which contained Device 1; their absorbance was monitored over time and then the rate of growth compared to bacteria that do not contain Device 1. The graph clearly shows that inserting Device 1 into cells considerably slows their growth in log phase compared with the growth of cells without Device 1 but the lag phase is shorter than in the bacteria without the inserted device. The key difference however is that bacteria containing Device 1 don't grow to as high optical density in log phase as the bacteria without Device 1.
Restriction DigestsText here
Plate Imagingtext here
Membrane Stress - Hydrophobic StressFile insert, right aligntext here Membrane Stress - Changes in pHtext here
Membrane Stress- Atomic Force MicroscopyAtomic Force Microscopy utilises Van-Der-Waals forces to attract the tip towards a surface, causing the bending of a cantilever, onto which a laser is pointed. The tip deflection causes the laser light to also be deflected and this is tracked using a bank of photodiodes. AFM is typically used to image surfaces at the atomic scale, as well as for Force Spectroscopy. When used in Force Spectroscopy, the tip is brought into direct contact with the sample, allowing the sample protein to bind to the silicon tip. Then, the AFM is slowly retracted at varying forces or to varying distances, applying a force to the protein. This causes portions of the protein to unravel, and the distinct patterns associated with α-helices and β-pleated sheets can be seen in this data. This allows the exact secondary and tertiary structure of a protein to be determined, but also the energy profile of various conformations, which in turn tells us how the protetin folds. For Bio-Device 1 however, the team wanted to measure how much force was required to trigger the stress activated Cpx Pathway. This was to be done by observing the culture under the fluoroscope portion of the F-AFM, and then "prod" a bacterium at a low force, slowly increasing the stress until a fluorescence response was seen. This would then give a direct measure of the stress threshold for Cpx, a value currently unknown in the literature. However, as with all new experiments, there were some teething issues. Firstly, it can be seen from the image that the culture were already fluorescing a little, likely due to smaller stresses in solution, although some can clearly be seen in the middle of binomial fission, which is known to also activate the pathway. The other issue was with trying to keep the bacterial cells stable enough to continually poke them with AFM tip. Normally, this sort of experiment is performed on Giant Unilamellar Vesicles and microbubbles about 5 micron wide. These microstructures are naturally forming precursors to cells, and thus of great interest to bio-physicists. However, as trying to poke a structure 5 microns wide with a tip only 40nm at its widest is akin to throwing a needle from space onto a ship in the Atlantic ocean, experimenters usually adhere the bubbles to the surface with a binding peptide. This could not be done for bio-device 1 without potentially triggering the pathway, so instead, it was hoped a more viscous solution and some micro-troughs scratched into the glass slide surface would be enough to keep them steady. Again though, bacteria are very different to inert bubbles of lipids, and tend to forcibly move themsleves regardless, hence results remained inconclusive. The approach showed some promise however, and it is hoped further work will be undertaken on this at a later date. In the meantime however, it is clear that on the microscale, there is always some fluorescence, so a background reading will always be necessary to make use of Micro-Beagle. Additionally, it may be worth using evolutionary development techniques to mutate a strain with a higher threshold tolerance.
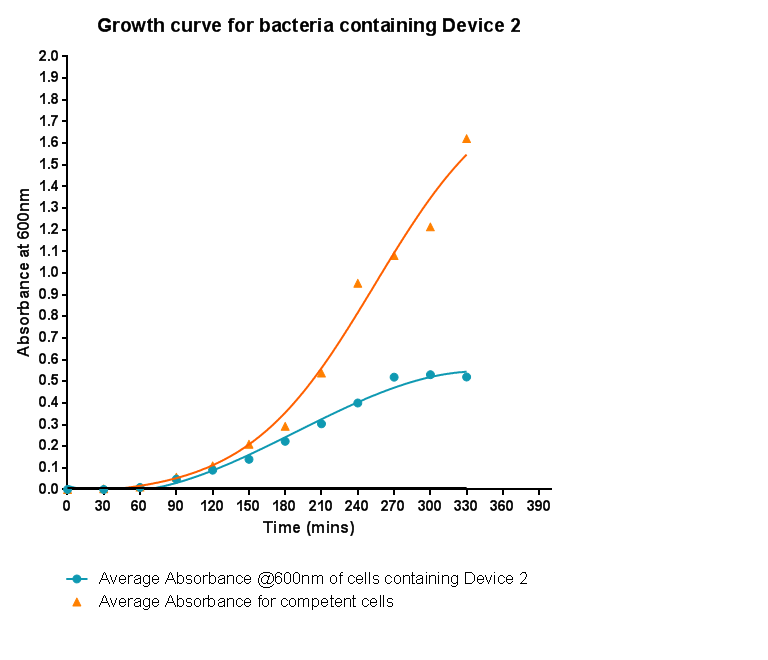
Characterisation of Device 2Growth CurveHere the growth of cells containing device 2 was monitored via absorbance and compared to the growth of cells that do not contain device 2. As was seen when device 1 was inserted into the cells, the growth is slowed down considerably. Although the graph shows a log and a lag phase for the cells containing Device 2, the absorbance reached in log phase is very low compared to cells without Device 2; it is even lower than the absorbance reached for cells containing Device 1.
Restriction Digeststext here
Plate Imagingtext here
Binding assay-washing beadsFile insert, right aligntext here
Binding assay-Confocal MicroscopyFile insert, left alignText here
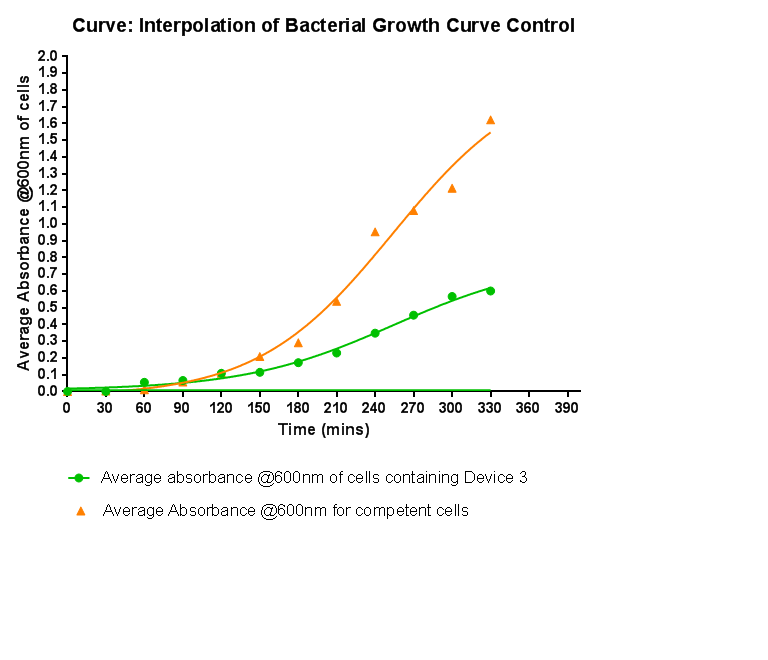
Characterisation of Device 3Growth CurveThis graph shows the results of monitoring the growth of cells with contain Device 3. As with cells containing Device 1 and Device 2, the growth of the cells is considerably slowed down when compared with cells that do not contain any device at all. A slight change in the gradient of the graph can be seen showing the transition of the cell growth from lag to log phase, so the cells are still growing normally, but the insertion of Device 3 is restricting the growth of the cells.
Restriction Digeststext here
Plate Imagingtext here
Binding Assay-Beads cause fluorescence?File insert, right aligntext here
| |||||||
 |
| ||||||

| |||||||

| |||||||
 "
"