Team:Paris Saclay/Notebook/August/30
From 2013.igem.org
Notebook : August 30
summary
- check the size by Gel electrophoresis for PCR clonies on Gibson assembly and ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3 .
- Do ligation for For promoter fnr(repressor)+ RBS_LacZ+Term_PSB1C3 , promoter fnr(activator)nirK + RBS_LacZ+Term_PSB3K3 and promoter fnr(repressor) + RBS_LacZ+Term_PSB3K3 and Transformation and
incubation.
lab work
- A.aero/anaerobic regulation system
1 -Gel electrophoresis of PCR clonies on Gibson assembly .
| [[]] |
|
2 -Gel electrophoresis of ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3 .
| [[]] |
|
- Ligation
- For promoter fnr(repressor)+ RBS_LacZ+Term_PSB1C3 :
| promoter fnr(repressor) | 8µl |
| RBS_LacZ+Term | 3µl |
| Ligation buffer | 2µl |
| Ligation T4 enzyme | 2µl |
| H2O | 6µl |
Transformation theligation and spread in LB plates with Chlorenphenicol and Xgal. incubated at 37°C in aerobic condition over night.
- For promoter fnr(activator)nirK + RBS_LacZ+Term_PSB3K3 :
| promoter fnr(activator)nirK | 3µl |
| RBS_LacZ+Term | 3µl |
| Plasmid PSB3K3 | 5µl |
| Ligation buffer | 2µl |
| Ligation T4 enzyme | 1µl |
| H2O | 6µl |
Transformation theligation and spread in LB plates with k and Xgal. incubated at 37°C in anaerobic condition over night.
- For promoter fnr(repressor) + RBS_LacZ+Term_PSB3K3 :
| promoter fnr(repressor) | 3µl |
| RBS_LacZ+Term | 3µl |
| Plasmid PSB3K3 | 5µl |
| Ligation buffer | 2µl |
| Ligation T4 enzyme | 1µl |
| H2O | 6µl |
Transformation theligation and spread in LB plates with k and Xgal. incubated at 37°C in aerobic condition over night.
| Previous week | Back to calendar | Next day |
Notebook : August 23
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
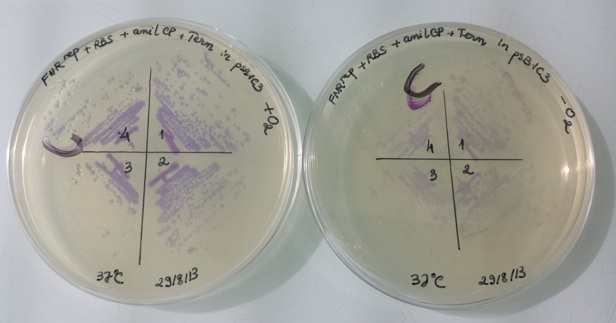
1 - Results of liquid culture of Pfnr-RBS-Amil CP-Term in PSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
IT WORKS !!! |
In anaerobic conditions, thank to promotor RBS-Amil CP-Term, the dectetion gene is activated and we can see a violet coloration of bacterias.
In aerobic conditions, promotor RBS-Amil CP-Term is inactive, the dectetion gene isn't activated and we can't see any coloration of bacterias.
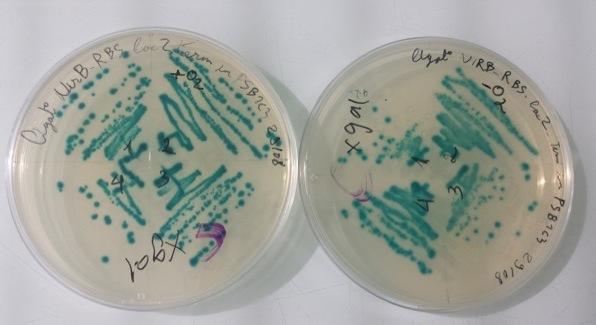
2 - Results of culture of Pfnr-RBS-Amil CP-Term in PSB1C3, NirB with RBS-LacZ-Term in PSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
Purification of 08/22/13 didn't work. We have blue colonies for Pfnr with RBS-Amil CP-Term in PSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for NirB with RBS-LacZ-Term in PSB1C3 in anaerobic conditions. |
3 - Ligation of Pfnr with RBS-LacZ-Term in PSB1C3
XiaoJing
Used quantities :
- Pfnr : 8µL
- RBS-LacZ-Term in PSB1C3 : 3µL
- Buffer ligation : 2µL
- Ligase : 1µL
- H2O : 6µL
4 - Transformation of ligation of Pfnr with RBS-LacZ-Term in PSB1C3 in DH5α
XiaoJing
Protocol : Bacterial transformation
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
1 - Electrophoresis of Colony PCR of FNR, RBS-FNR and RBS-BphR2
| ]] |
|
Expect sizes :
- FNR : 1096 bp
- RBS-FNR : 1014 bp
- RBS-BphR2 : 1469 bp
|
We obtain fragment at right size for FNR and RBS-FNR. The Gisbon assembly of 08/26/13 was good. |
 "
"