Team:NYMU-Taipei/Project/Enter
From 2013.igem.org


Contents |
Introduction
In this part, the main purpose is to send Bee. coli into bees and make sure it can survive in the midguts. Therefore, we chose MG1655 as the chassis of our circuit, then incorporating MG1655 into alginate microcapsules, and fed bees with sugar water containing microencapsulation beads. According to our experiment, it could successfully transport the engineered Bee. coli into the bees’ midgut.
Background
Why did we choose MG1655
- The main reason we want to choose one of the substrain, MG1655, of E. coli K12 strain as our carrier is E. coli K12 strain exists in the midgut's of honeybees naturally. In addition, compared to nother substrain of k-12, MG1655 was selected for having few genetic manipulations from the archetypal E. coli K-12 strain, and it has already been well-studied as well.
- The second reason for choosing E. coli K12 strain is that what we utilize to repress the sprouting of Nosema (Mannosidase), sense the invasion of Nosema (ROS) and kill Nosema (Defensin and Abaecin) are harmful to the microbes. Notably, they are naturally produced in bees as well. Besides, the immune system of honeybee will prevent our E. kobee colonizing in the epithelial cells of guts. To solve these problems, we select MG1655 which naturally exists in bees.
- Therefore, MG1655 is supposed to survive in the midguts after we send it into bees by encapsulation.
- However, the transformation efficiency of MG1655 is not as good as that of DH5 alpha, which is another substrain of K12. On the other hand, the oxidative stress tolerance of MG1655 is much better than DH5 alpha theoretically because it's more similar to wild-type.
Why do we encapsulate Bee. coli?
The host immune system imposes a great threat on our engineered Bee. coli. Besides we have to transported our engineered Bee. coli in the bees through bees’ digest system, which may contain many kinds of digestive solution with variable pH value that do harm to our engineered Bee. coli.
To solve this problem, we have to cover the Bee. coli with a membrane. And encapsulation is an easy way to reach the goal. Comparing to other approaches, cell encapsulation is easy to manipulate, so we choose cell encapsulation as the approach to cover Bee. coli.
There are many different biomaterial for cell encapsulation. Among those biomaterial, we choose alginate as our encapsulation material for its abundance, excellent biocompatibility and biodegradability properties.
How to get capsule in
We have considered many methods to get capsule into Bees’ midgut, such as spraying the water mixed with microencapsulation of E.coli into the hives. Considering bees’ habits, we choose the way to mix microencapsulation of Bee. coli with the food of bees( sugar water) for that
How to keep bees alive out of the hives
We constructed a device to keep the bees alive when they were out of the hives. The device contained a cup and an eppendorf. The cup was the main part which bees active, with many pores on the top to exchange the air. The eppendorf is attached next to the cup, with a special straw to supply the sugar water. The sugar water in the eppendorf is the food of bees. By mixing the microencapsulation of Bee. coli into the sugar water, we could easily help the bees to intake Bee. coli.
Experiment
Comparison of DH5 alpha and MG1655
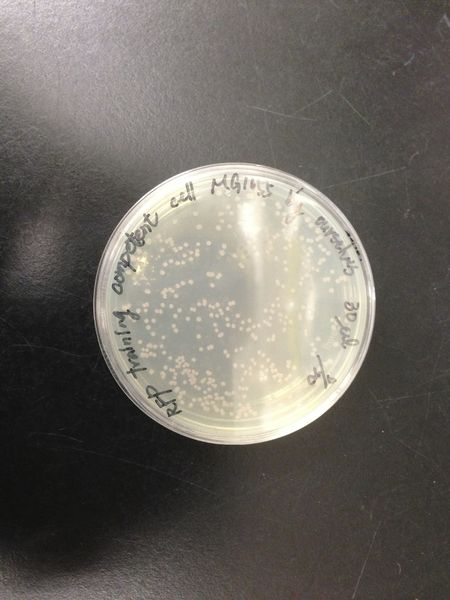
We transform red fluorescent protein into MG1655 and DH5 alpha, then spreading on the LB plates. After cultured at 37 degree for 16 hours, we calculated the number of colonies and found the transformation efficiency of DH5 alpha is about 100-fold higher than MG1655.
| DH5 alpha | MG1655 |
|---|---|
Oxidative stress tolerance of MG1655
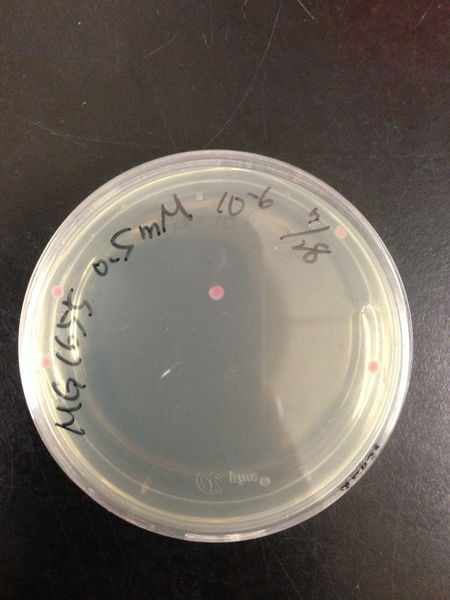
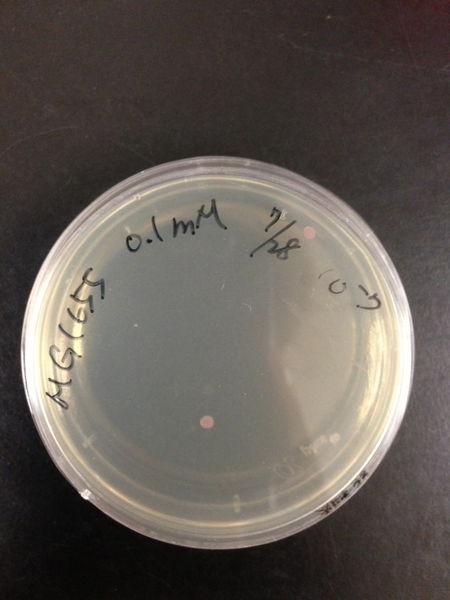
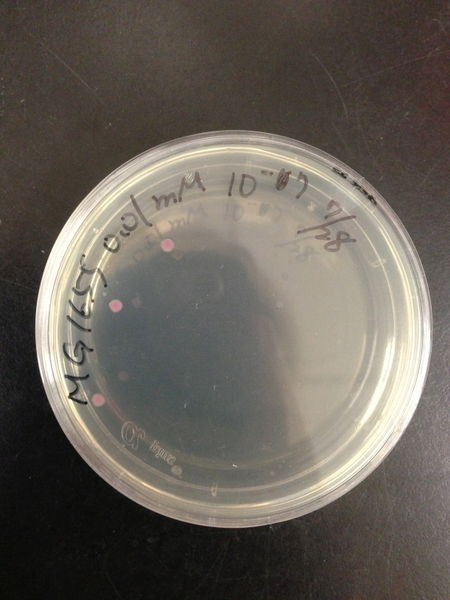
To know the oxidative stress tolerance of MG1655, we test the susceptibility to H2O2 in MG1655.
< Survival test >
- MG1655 cells were cultivated at 37 ˚C until the optical density (OD600) of the medium became 0.4~0.6
- The culture was equally dispensed (1 ml each) into sample tubes, and then cells were treated with various concentrations of H2O2 (final concentrations were as follows: 10μM, 50μM, 100μM, 500μM, 1 mM, 5mM, 10mM, 50mM and 100mM) at 37 ˚C for 60 min
- Testing the OD of each samples
- Doing serial dilution on samples by mixing fresh LB medium with samples
- 20μl of the diluted sample was spread out on a LB plate (amp-) and incubated at 37 ˚C (overnight)
< Result and discussion >
| Susceptibility to H2O2 in MG1655 shown in OD | Susceptibility to H2O2 in MG1655 shown in colonies |
|---|---|
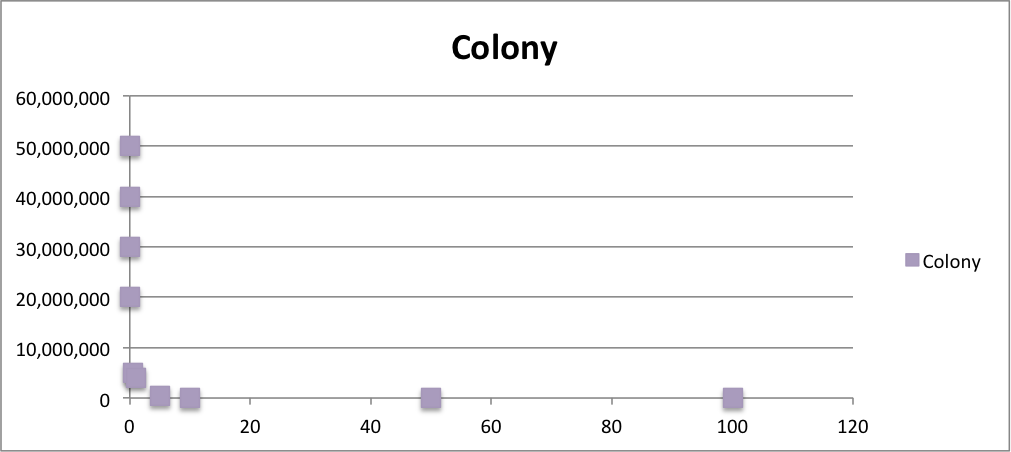
From these charts, we know the oxidative stress tolerance of MG1655 is higher than 5mM, and epithelial cells is only 0.05mM. In the other words, the tolerance to ROS of MG1655 is over 100-fold higher than cells in midgut. Therefore, MG1655 will survive even in the stress environment in the midgut.
| Treated with 100mM H2O2 diluted 104times | Treated with 50mM H2O2 diluted 104times | Treated with 10mM H2O2 diluted 105times | Treated with 5mM H2O2 diluted 105times | Treated with 1mM H2O2 diluted 106times |
|---|---|---|---|---|
| Treated with 500μM H2O2 diluted 106times | Treated with 100μM H2O2 diluted 107times | Treated with 50μM H2O2 diluted 107times | Treated with 10μM H2O2 diluted 107times | Treated with 0μM H2O2 diluted 107times |
Comparison table of MG1655 and DH5 alpha
| Comparison | MG1655 | DH5 alpha |
|---|---|---|
| Strain | K12 | K12 |
| Genotype | F-, lambda-, rph-1 | -fhuA2 lac(del)U169 phoA glnV44 Φ80' lacZ(del)M15 gyrA96 recA1 relA1 endA1 |
| Characteristics | Similar to wild-type | Designed for transformation |
| Transformation efficiency | >1x108cfu/μg | >1x106cfu/μg |
| Oxidative stress tolerance | <0.1mM | >5mM |
Encapsulation test
We carried out an experiment to see if microencapsulation of Bee. coli can really help our engineered Bee. coli enter bees’ midguts. We assume that only when engineered E .coli protected by the alginate membrane, it can be transported to bees’ midguts.
Before the main experiment, we have to create microencapsulation of E. coli. Cell Encapsulation is technically achieved by dropping a mixture of cells and liquid alginate into a calcium chloride solution which solidifies the droplets, transforming them into the hydrogel beads. We should control beads’ size within the range of 20~100μm to ensure the beads can be intake by bees. We use a machine, which can help us achieve this goal.
Next we separated our bees into three categories, feed them with sugar mixed with microencapsulation of E. coli, sugar mixed with E. coli without encapsulation and sugar only. The E. coli is engineered with GFP gene, so we could find it easily under microscope. After three days, we pulled out the bees’ midguts and see if there is any engineered E. coli with GFP. As we expected, E. coli only exited in the midgut of those who had intake sugar mixed with microencapsulation beads of E.coli.
Brief Result
After we proved that microencapsulation beads of E. coli could be sent into midgut through bees’ digestion system, we came up with an idea that we can create a product which is the mixture of sugar and microencapsulation beads of engineered Bee. coli. So we mixed the solution of microencapsulation with sugar powder and dry it. Then, we create the drug powder which can cure CCD.
 "
"