Team:NYMU-Taipei/Project/Inhibition/Id-Nosema
From 2013.igem.org


Contents |
Introduction
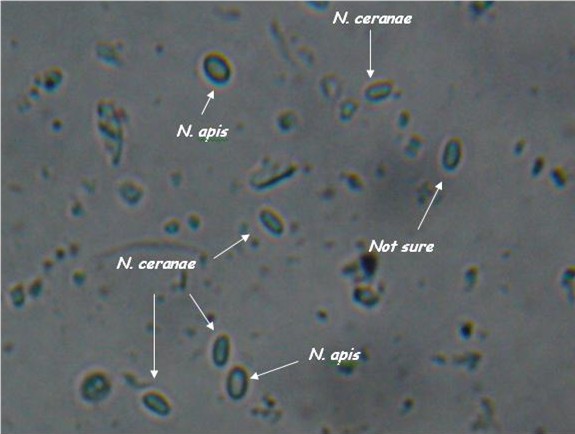
The identification of N. apis and N. ceranae is important and still need further research. So far the best differentiation method is the use of species–specific primers; however, the sequence differences are limited to the ribosomal RNA loci, leading to overestimation of Nosema infection rate. Thanks to recent genome study of N. apis and N. ceranae, we proposed novel primers for differentiation of N. spp. based on highly conserved, species–specific protein coding genes rather than species–specific sequences in rRNA genes, which is likely to improve the accuracy of species identification.
Background
N. spp. Differentiation Methods
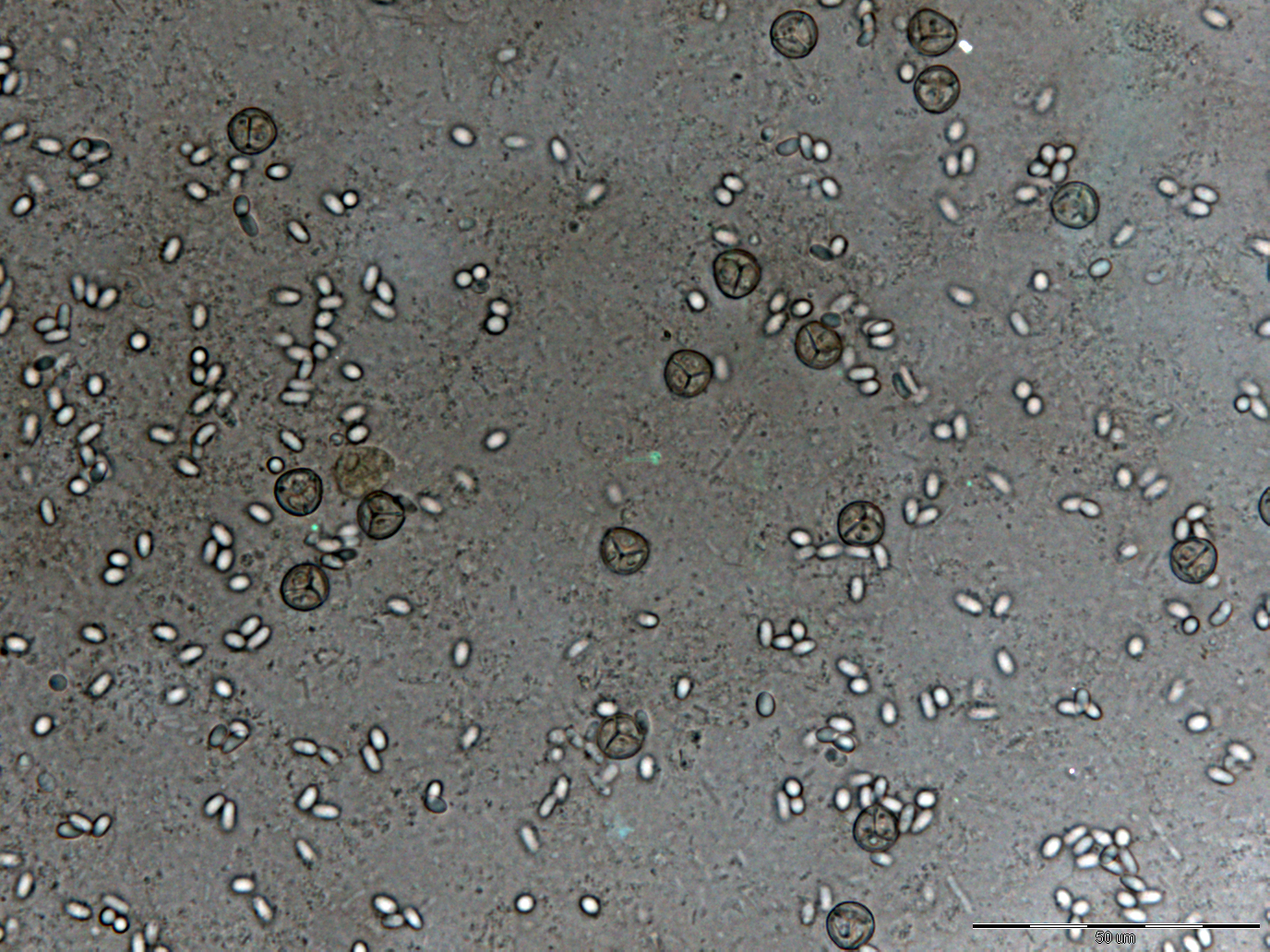
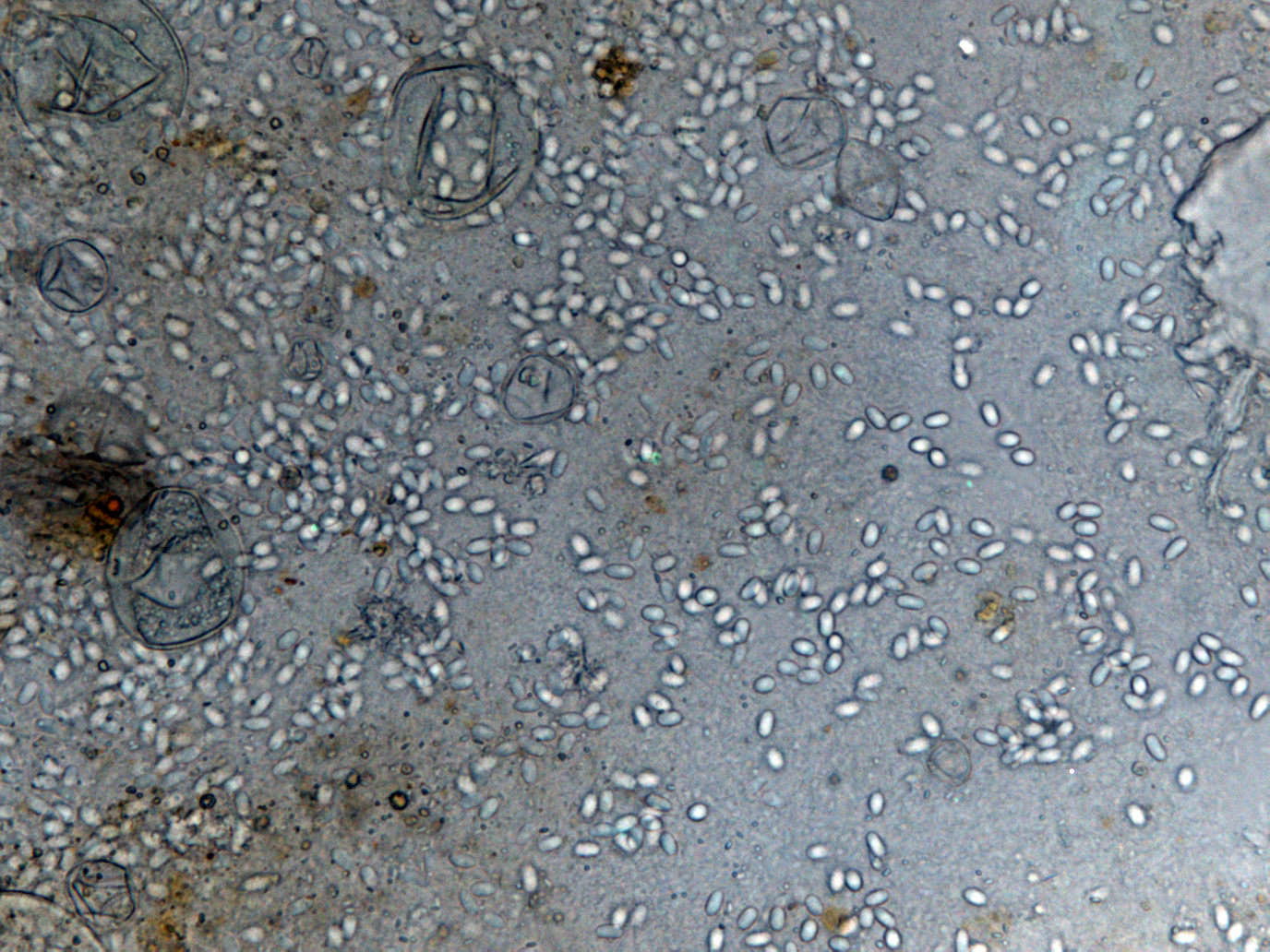
- Light microscopy method: It is based on morphology difference of N. apis and N. ceranae, revealing that spores of N. ceranae are oval or rod shaped like a rice, and also slightly thinner than N. apis. Light microscopy method is convenient but not scientific enough since N. apis bears a striking resemblance to N. ceranae.
- Molecular methods: Species-specific primers are more accurate than light microscopy method. The problem is that so far nearly all protocols based on species–specific sequence differences are limited to the ribosomal RNA loci, which may lead to confusing results due to polymorphisms in and recombination between the multi-copy 16S rRNA genes, and hence overestimate the rate of Nosema infections.
- Our new primers: This year, we created better primers based on recently published, highly conserved and absolutely species–specific sequence differences in protein coding genes. To be more specific, the primers are based on species–specific genes instead of species–specific sequences. As more and more genes of Nosema spp. are revealed, we are able to choose the genes that are not homologs N. apis and N. ceranae for new primers. Our new primers are confirmed by BLAST through nucleotide blast and tblastn using Nucleotide collection (nr/nt) database and Whole-genome shotgun contigis (wgs) database. We propose that our new primers should become the preferred molecular tool for differentiation of Nosema spp. and thus assist in apiculture.
Experiment
Primer Design
1.Common gene of N. spp.--- NcORF-00182 hypothetical protein & NAPIS_ORF01775 chitin synthase d
NcORF-00182
Product=1089bp
NAPIS_ORF01775
Product=1103bp
2.Specific gene of N. ceranae---NcORF-00714 hypothetical protein
Product=861bp for N. ceranae
3.Specific gene of N. apis---NAPIS_ORF02138 class iv chitinase
Product=1320bp for N. apis
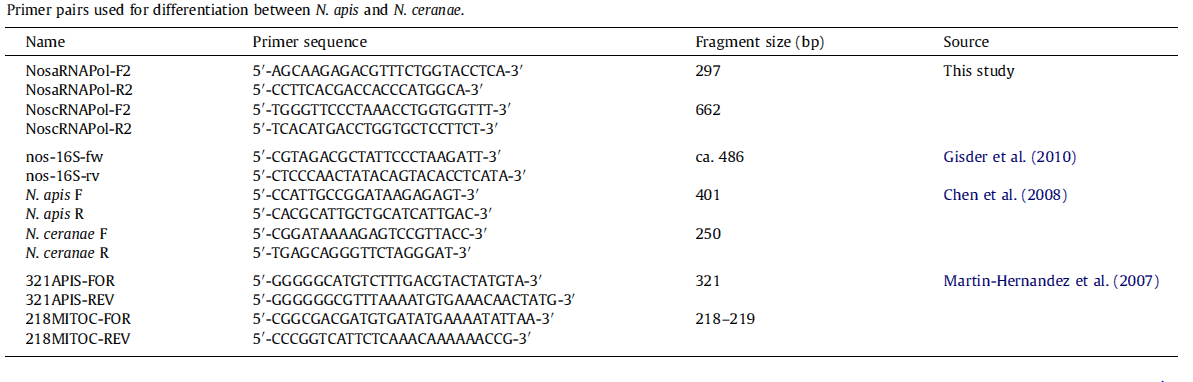
Besides testing our new primers using real PCR, we also chose traditional primers to double-check whether the bees were infected with N. app.
Traditional Primer
N. ceranae
forward primer: cggataaaagagtccgttacc-30 reverse primer: tgagcagggttctagggat Product=250bp for N. ceranae
N. apis primers
forward primer: ccattgccggataagagagt reverse primer: cacgcattgctgcatcattgac Product=401bp for N. apis 4. The primer pairs, Nosema F/R, N. apis F/R, and N. ceranae F/R generated PCR fragments of 208, 401, and 250 bp, respectively.
Results
Results of Traditional Primers
The bees were infected with both N. apis and N. ceranae. Another thing worth mentioning is that two bands instead of one band appeared when checking the existence of N. apis. The next thing we are planning to do is to compare the outcomes of our new primers with traditional ones to verify the feasibility of the novel method.
 "
"