Team:Paris Saclay/Notebook/August/5
From 2013.igem.org
Contents |
Notebook : August 5
Lab work
A - Aerobic/Anaerobic regulation system
Obtaining the PSB3K3 backbone plasmid
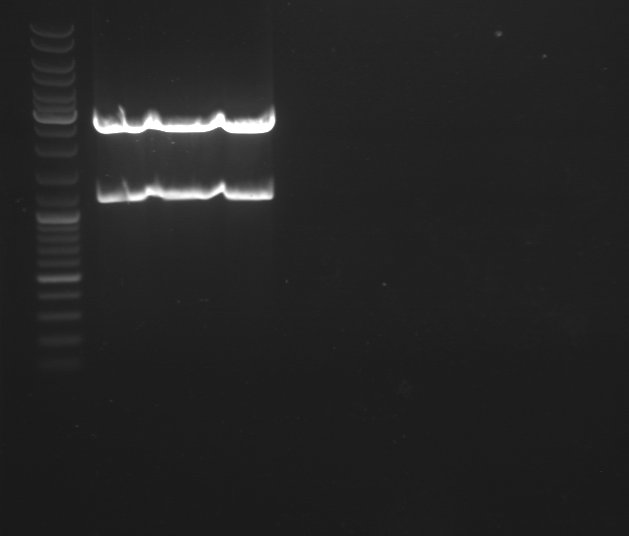
1 - Electrophoresis of BBa_J004450 digested by EcoRI/Pst1 to check if the digestion was right
Damir, Nadia
| IMAGE |
|
Expected sizes :
- PSB3K3 : 2750kb
- GFP : 1069kb
We obtained fragments of the right size. Now we can purify the PSB3K3 plasmid.
2 - Gel purification of the PSB3K3 plasmid from BBa_J004450 digested by EcoRI/Pst1
2.1 - Migration of the remaining 45µL of BBa_J004450 digested by EcoRI/Pst1
Anaïs
|
|
Now we can do a gel purification of the highest band which contains the PSB3K3 plasmid.
2.2 - Electroelution of the highest band to extract the PSB3K3 plasmid from the gel
Nadia
Protocol : Electroelution
We let the plasmid precipitate during the night.
Obtaining RBS_LacZ+Term_PSB1C3
1 - Colony PCR on e.coli with RBS_LacZ+Term_PSB1C3 for 25 colonies
Anaïs
- Colony counting :
- Low concentration petri dish : 47 colonies
- High concentration petri dish : 145 colonies
- Picking of 25 colonies
- Preparation of 700µL of Master mix
- H2O : 590µL
- dNTP : 28µL
- VF2 primer : 3.5µL
- VR primer : 3.5µL
- DreamTaq buffer 10x : 70µL
- DreamTaq enzyme : 5µL
Protocol : Colony PCR
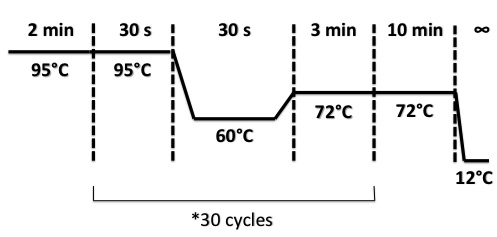
PCR Program :
2 - Gel electrophoresis of the colony PCR products
Anaïs, Damir

|
|
Expected size : 3583bp
Colonies 10, 14, 15 exhibit plasmids with the right length.
3 - PCR product (made the 08/01/2013) purification
Damir
available quantity:
- FNR Part1 : 10 µl
- FNR Part2 : 19 µl
- RBS FNR Part1 :16.1µl
- RBS BphR2 Part1 : 28µl
- BphR2 Part1 : 16.4 µl
- BphR2 Part2 : 18.9 µl
Protocol : kit purification
Manipulation error : The elution step was made using the recuperation tube from the filtering step, instead of a new, clean eppendorf tube.
 "
"