Team:DTU-Denmark/Experiment2
From 2013.igem.org
(→Methods) |
(→Introduction) |
||
| (66 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{:Team:DTU-Denmark/Templates/StartPage| | + | {{:Team:DTU-Denmark/Templates/StartPage|Anaerobic production of nitrous oxide from nitrite}} |
__TOC__ | __TOC__ | ||
| - | == | + | == Introduction == |
| - | + | In this anaerobic experiment we characterize how readily nitrite is converted to nitric oxide (NO), and subsequently to nitrous oxide (N<sub>2</sub>O) by adding nitrite (NO<sub>2</sub><sup>-</sup> ) in a series of spikes to: | |
| + | * an untransformed strain of ''E. coli'' | ||
| + | * an untransformed strain of ''P. aeruginosa'' | ||
| + | * Mutant 2: a strain of ''E. coli'' transformed with the Nir gene from ''P. aeruginosa'' | ||
| - | + | [[File:dtu-Mutant2-extra_ws.png|right]] | |
| + | We also monitor nitrite, ammonia and nitrate concentrations before each spike. The concentrations of nitrous oxide is measured continually and the ammonia, nitrite and nitrate concentrations are measured at a series of time points around the spike. For some experiments, we have data for the concentration of nitric oxide as well, however, the sensor frequently gave unreliable results and was replaced during the summer. While we were waiting for the new one to arrive, we were not able to take NO measurements. | ||
| - | + | ==Methods== | |
| - | + | ||
| - | + | The experimental procedure follows [[Team:DTU-Denmark/Methods/Determining_concentration_of_nitrogen_compounds/Experiment_2|Protocol for Experiment 2]]. | |
| - | + | For the calibration of the N<sub>2</sub>O and NO electrodes we follow the [[Team:DTU-Denmark/Methods/Calibrating_Electrodes#Calibration_of_NO_and_N2O_electrodes.2Fprobes|calibration protocol]]. | |
| - | + | == Results and Discussion == | |
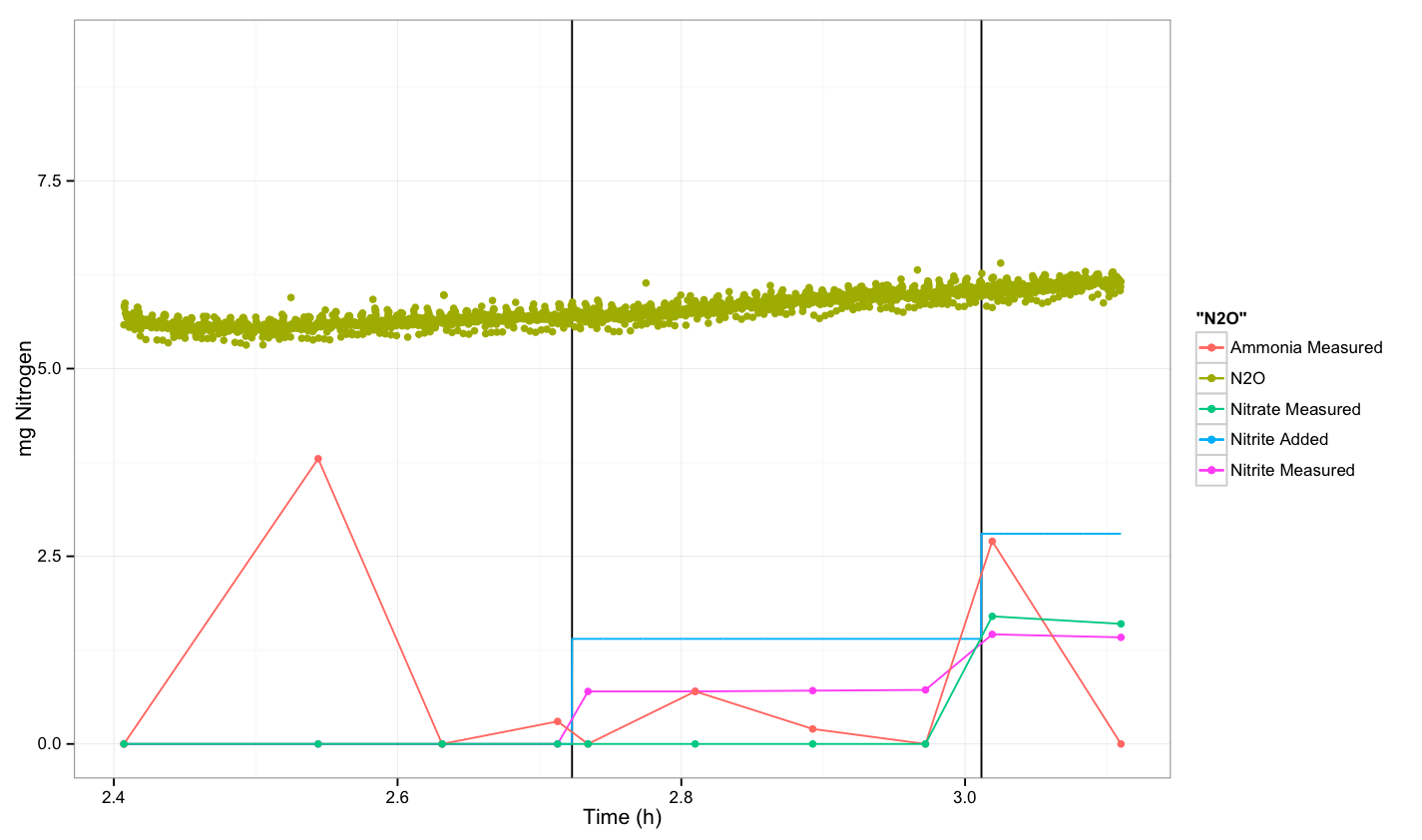
| - | + | In the following diagrams, the y-axis shows mg of Nitrogen (as nitrite, nitrous oxide or other N-source) per litre vs time. Vertical lines indicate the time at which we spiked with nitrite. All experiments were performed anaerobically. | |
| - | + | === Control: Untransformed ''E. coli'' -- Round 1 === | |
| - | + | The first round of the experiment was performed on [[Team:DTU-Denmark/Notebook/8_August_2013#Lab_115| August 8<sup>th</sup>]] and [[Team:DTU-Denmark/Notebook/9_August_2013#Lab_115| August 9<sup>th</sup>]]. | |
| - | + | ||
| - | + | ||
| - | + | It appears that we observed a response from the cells after the third spike. However, this could also be due to fluctuations in the sensor. | |
| + | [[File:DTU-Experiment-2-results.png|600px]] | ||
| - | + | === Control: Untransformed ''E. coli'' -- Round 2 === | |
| - | + | We repeated the experiment to try to reproduce the response that we saw in round 1. The experiment was performed on [[Team:DTU-Denmark/Notebook/22_August_2013#lab_115| August 22<sup>th</sup>]]. We were not able to see a response in either replicate that we did this round. At the end of the second replicate, the sensor gave a jump in reading, but due to the quick drop off, it is most likely that this is due to fluctuation in the sensor and is not a reliable reading. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[File:Dtu Experiment 2 round 2 --sample A.png|600px]] | |
| + | [[File:Dtu Experiment 2 round 2 -- sample B.png|600px]] | ||
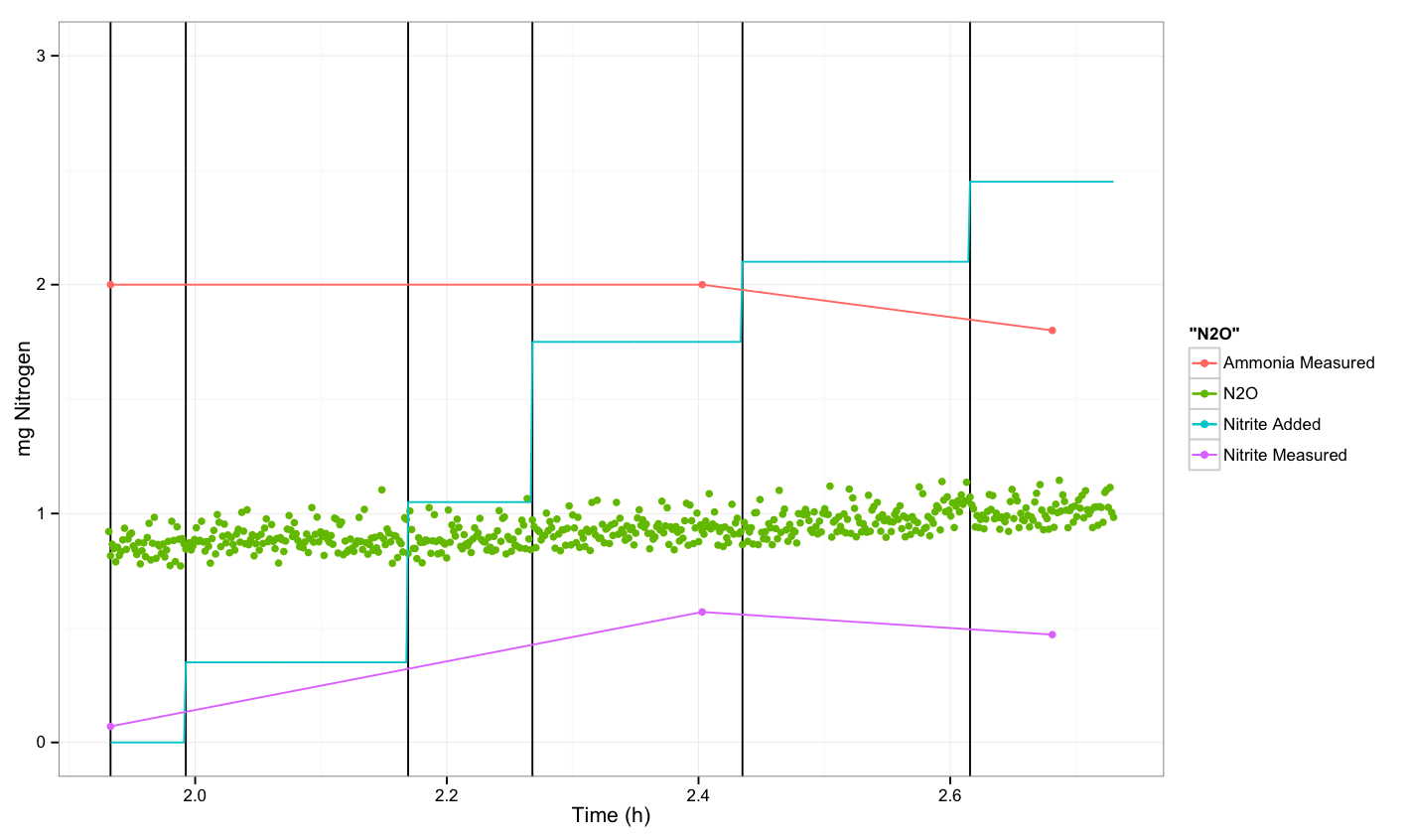
| - | + | === Untransformed ''P. aeruginosa'' === | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | We also did not see a response for the P. aeruginosa, which we would expect. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | The experiment was performed on [[Team:DTU-Denmark/Notebook/7_August_2013#Lab_115|August 7<sup>th</sup>]]. | |
| - | + | ||
| + | [[File:Dtu pseudo exp.png|600px]] | ||
| - | + | === Nir Transformed ''E. coli'' === | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
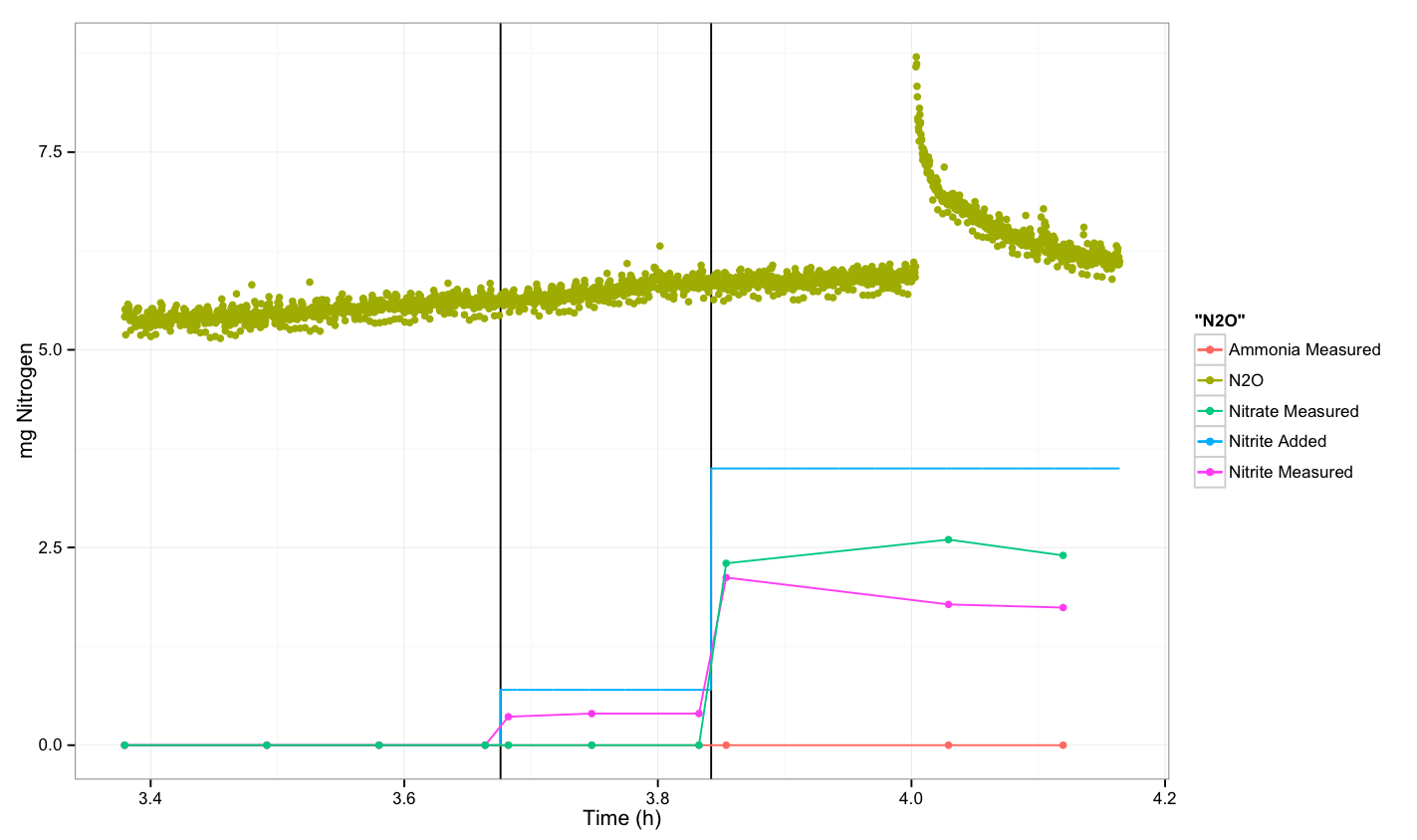
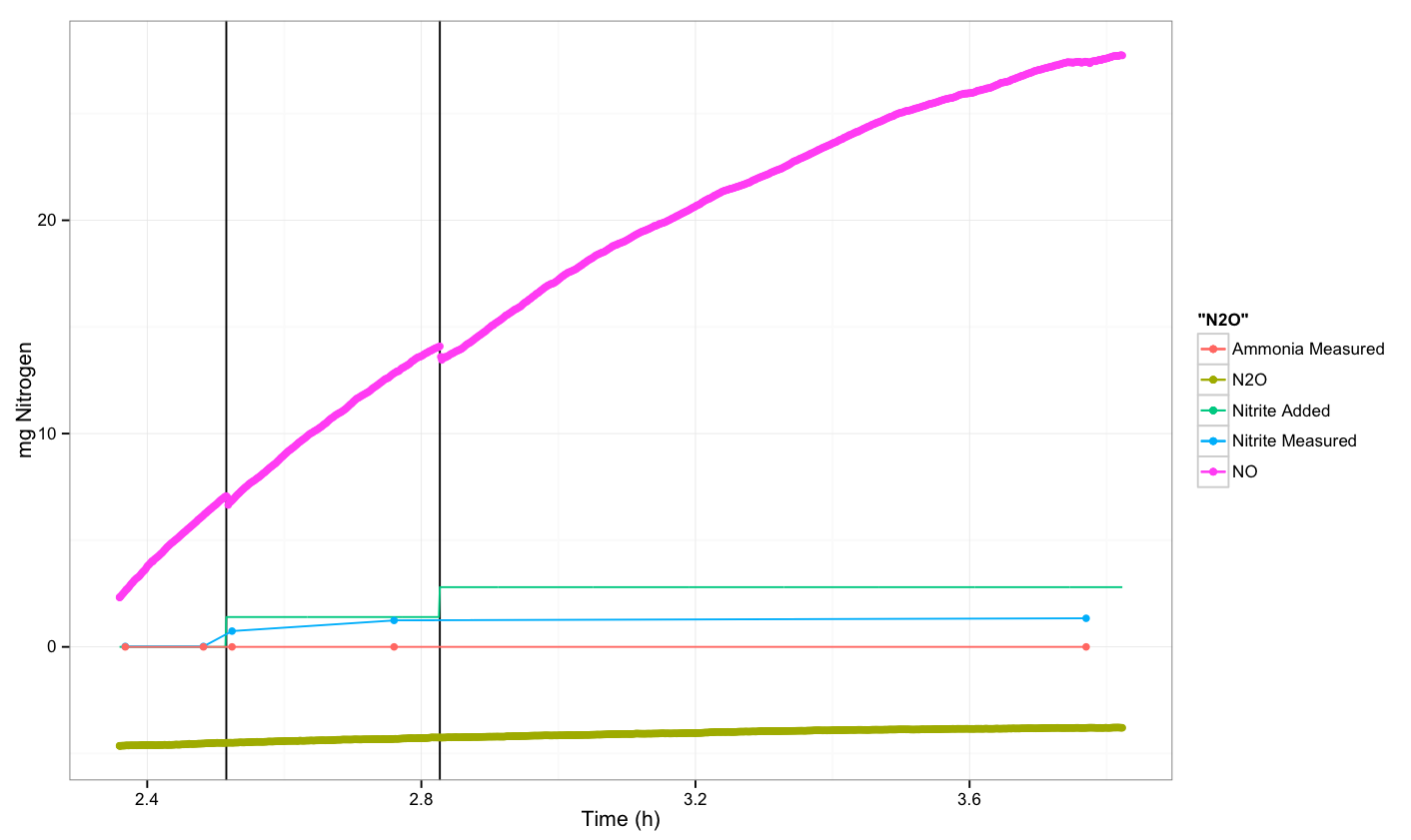
| - | + | Finally, we measured both N<sub>2</sub>O and NO, but did not see an increase in N<sub>2</sub>O. The NO sensor did not stabilize over the course of the experiment, and these results are not realistic. Additionally, the N<sub>2</sub>O sensor was calibrated correctly, but when the experimental readings were converted to mg nitrogen, the values were negative. Since this is not realistic, it is likely that the readings are not accurate and that possibly the sensor should be replaced. Unfortunately, we did not have enough time to repeat the experiment with a new sensor. | |
| - | + | ||
| - | + | The experiment was done on the 19th of September and the procedure is described in [[Team:DTU-Denmark/Notebook/19_September_2013|our notebook for September 19<sup>th</sup>]]. | |
| - | [[File: | + | [[File:Dtu-nir.png|600px]] |
| + | |||
| + | <div style="clear: both;"></div> | ||
| + | |||
| + | == Conclusions == | ||
| - | + | Despite multiple replicates over multiple experiments, we were not able to reliably detect nitrous oxide being produced in any strain as a result of the addition of nitrite. | |
| - | {{:Team:DTU-Denmark/Templates/ | + | {{:Team:DTU-Denmark/Templates/EndPage}} |
Latest revision as of 12:37, 4 October 2013
Anaerobic production of nitrous oxide from nitrite
Contents |
Introduction
In this anaerobic experiment we characterize how readily nitrite is converted to nitric oxide (NO), and subsequently to nitrous oxide (N2O) by adding nitrite (NO2- ) in a series of spikes to:
- an untransformed strain of E. coli
- an untransformed strain of P. aeruginosa
- Mutant 2: a strain of E. coli transformed with the Nir gene from P. aeruginosa
We also monitor nitrite, ammonia and nitrate concentrations before each spike. The concentrations of nitrous oxide is measured continually and the ammonia, nitrite and nitrate concentrations are measured at a series of time points around the spike. For some experiments, we have data for the concentration of nitric oxide as well, however, the sensor frequently gave unreliable results and was replaced during the summer. While we were waiting for the new one to arrive, we were not able to take NO measurements.
Methods
The experimental procedure follows Protocol for Experiment 2.
For the calibration of the N2O and NO electrodes we follow the calibration protocol.
Results and Discussion
In the following diagrams, the y-axis shows mg of Nitrogen (as nitrite, nitrous oxide or other N-source) per litre vs time. Vertical lines indicate the time at which we spiked with nitrite. All experiments were performed anaerobically.
Control: Untransformed E. coli -- Round 1
The first round of the experiment was performed on August 8th and August 9th.
It appears that we observed a response from the cells after the third spike. However, this could also be due to fluctuations in the sensor.
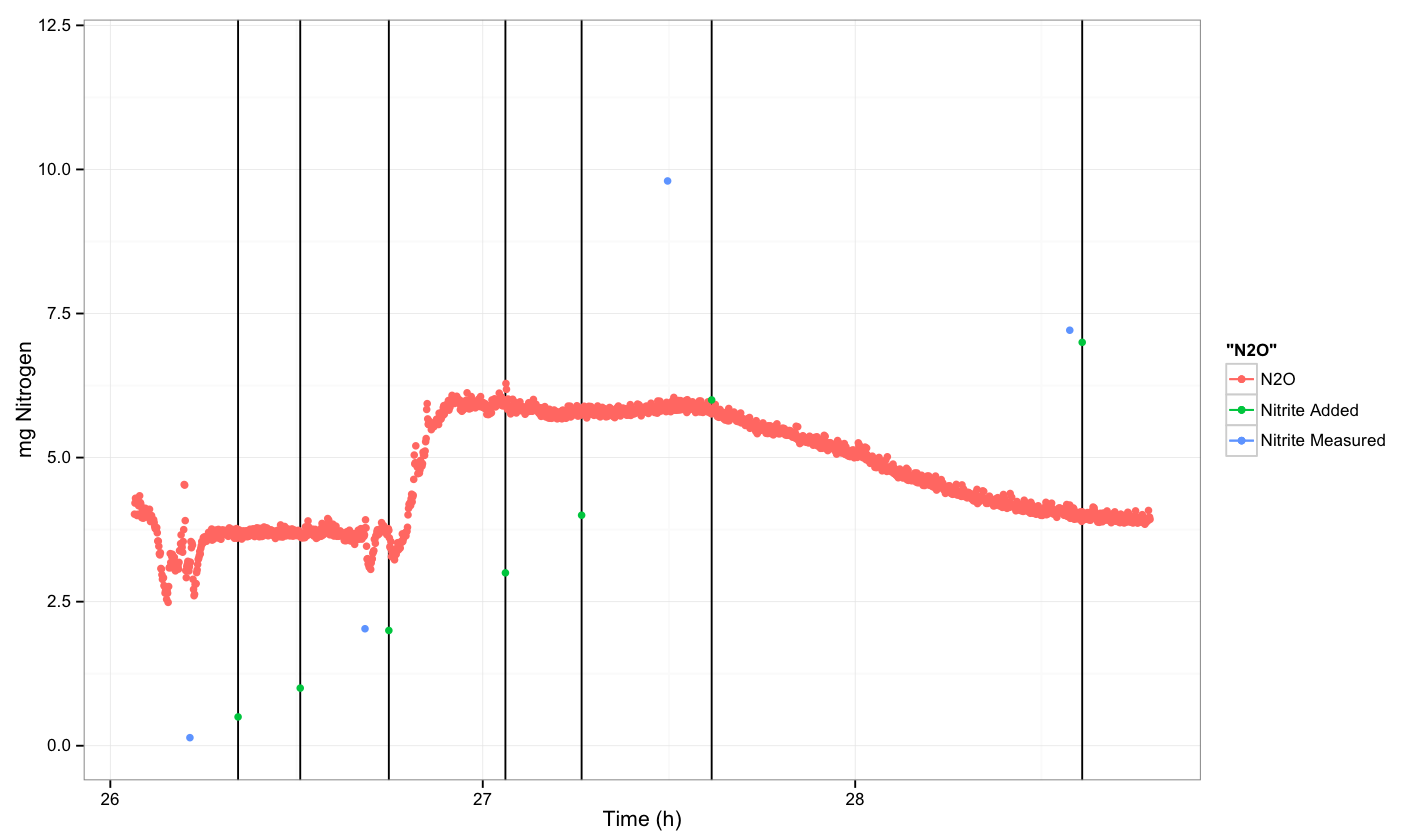
Control: Untransformed E. coli -- Round 2
We repeated the experiment to try to reproduce the response that we saw in round 1. The experiment was performed on August 22th. We were not able to see a response in either replicate that we did this round. At the end of the second replicate, the sensor gave a jump in reading, but due to the quick drop off, it is most likely that this is due to fluctuation in the sensor and is not a reliable reading.
Untransformed P. aeruginosa
We also did not see a response for the P. aeruginosa, which we would expect.
The experiment was performed on August 7th.
Nir Transformed E. coli
Finally, we measured both N2O and NO, but did not see an increase in N2O. The NO sensor did not stabilize over the course of the experiment, and these results are not realistic. Additionally, the N2O sensor was calibrated correctly, but when the experimental readings were converted to mg nitrogen, the values were negative. Since this is not realistic, it is likely that the readings are not accurate and that possibly the sensor should be replaced. Unfortunately, we did not have enough time to repeat the experiment with a new sensor.
The experiment was done on the 19th of September and the procedure is described in our notebook for September 19th.
Conclusions
Despite multiple replicates over multiple experiments, we were not able to reliably detect nitrous oxide being produced in any strain as a result of the addition of nitrite.
 "
"