Team:INSA Toulouse/contenu/lab practice/notebook/calendar/ribo
From 2013.igem.org
(Difference between revisions)
| Line 90: | Line 90: | ||
{ | { | ||
color: #5a6060;} | color: #5a6060;} | ||
| + | |||
| + | h2.faq { | ||
| + | display:list-item; | ||
| + | list-style-image : url(https://static.igem.org/mediawiki/2013/8/84/Insa-toulouse2013-listpuce1.png); | ||
| + | width:auto; | ||
| + | height:27px; | ||
| + | line-height:24px; | ||
| + | background: no-repeat; | ||
| + | border:none; | ||
| + | color:#5a6060; | ||
| + | padding:3px 0 0 0; | ||
| + | font-weight:400; | ||
| + | font-family:'Open Sans'; | ||
| + | margin:0 0 50px 0; | ||
| + | font-size:14px; | ||
| + | } | ||
| + | |||
| + | .infos {width:680px; margin:0 15px 50px 0;} | ||
| + | |||
| + | .infos ul{color:#5a6060; font-family:'Open Sans'; font-size:14px; line-height:28px; } | ||
| + | |||
| + | .infos .circlearrow li{ display : list-item; list-style-image : url(https://static.igem.org/mediawiki/2013/9/94/Insa-toulouse2013-listpuce2.png);} | ||
</style> | </style> | ||
| + | |||
| + | |||
| + | <script type="text/javascript" src="js/jquery.js"></script> | ||
| + | <script type="text/javascript"> | ||
| + | <!-- | ||
| + | $(document).ready(function(){ | ||
| + | // On cache les sous-menus : | ||
| + | $('div.accordeon div.infos').hide(); | ||
| + | // Curseur main sur les h2 | ||
| + | $('div.accordeon > h2.faq').css('cursor','pointer'); | ||
| + | $('div.accordeon > h2').click(function(){ | ||
| + | //si ouvert -> referme | ||
| + | if($(this).next('div.infos:visible').length!=0){ | ||
| + | $(this).next('div.infos').slideUp('normal'); | ||
| + | } | ||
| + | //si cache, on ferme les autres et on affiche | ||
| + | else { | ||
| + | $('div.accordeon div.infos').slideUp('normal'); | ||
| + | $(this).next('div.infos').slideDown('normal'); | ||
| + | } | ||
| + | return false; | ||
| + | }); | ||
| + | } ) ; | ||
| + | // --> | ||
| + | </script> | ||
| + | |||
| + | </style> | ||
| + | |||
<!--/* HTML Contenu*/--> | <!--/* HTML Contenu*/--> | ||
| Line 106: | Line 156: | ||
<div class="clear"></div> | <div class="clear"></div> | ||
| - | + | <div class="accordeon"> | |
| - | + | <h2 class="faq" title="Click to open the FAQ">July 2013</h2> | |
| - | + | <div class="infos"> | |
| - | + | ||
| - | + | ||
<ul class="circlearrow"> | <ul class="circlearrow"> | ||
<li><span class="spantitle2">Week 6 (15-21 July)</span><br> | <li><span class="spantitle2">Week 6 (15-21 July)</span><br> | ||
| Line 122: | Line 170: | ||
Assembling the riboregulators in pSB1C3 … again…<br> | Assembling the riboregulators in pSB1C3 … again…<br> | ||
</li> | </li> | ||
| + | </ul></li> | ||
</ul> | </ul> | ||
</li> | </li> | ||
| + | </div> | ||
| + | </div> | ||
| + | <div class="clear"></div> | ||
| + | |||
| - | < | + | <div class="accordeon"> |
| + | <h2 class="faq" title="Click to open the FAQ">August 2013</h2> | ||
| + | <div class="infos"> | ||
<ul class="circlearrow"> | <ul class="circlearrow"> | ||
<li><span class="spantitle2">Week 8 (29-4 August)</span><br> | <li><span class="spantitle2">Week 8 (29-4 August)</span><br> | ||
| Line 213: | Line 268: | ||
<br><br> | <br><br> | ||
</li> | </li> | ||
| + | </ul></li> | ||
</ul> | </ul> | ||
</li> | </li> | ||
| + | </div> | ||
| + | </div> | ||
| + | <div class="clear"></div> | ||
| + | |||
| - | < | + | <div class="accordeon"> |
| + | <h2 class="faq" title="Click to open the FAQ">September 2013</h2> | ||
| + | <div class="infos"> | ||
<ul class="circlearrow"> | <ul class="circlearrow"> | ||
<li><span class="spantitle2">Week 13 (1-8 September)</span><br> | <li><span class="spantitle2">Week 13 (1-8 September)</span><br> | ||
| Line 297: | Line 359: | ||
20/09 <br> Minipreps and 0,8%gel after EcoRI/PstI digestion : Two clonings failed. <br> | 20/09 <br> Minipreps and 0,8%gel after EcoRI/PstI digestion : Two clonings failed. <br> | ||
| - | |||
</li> | </li> | ||
| - | </ul> | + | </ul> |
</li> | </li> | ||
| - | </ | + | </div> |
</div> | </div> | ||
| + | <div class="clear"></div> | ||
<br> | <br> | ||
Revision as of 11:37, 27 September 2013
Calendar
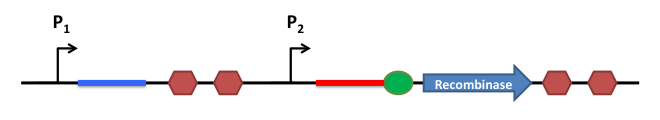
Riboregulation System
July 2013
- Week 6 (15-21 July)
19/07
Arrival of the synthesis.
20/07
Assembling the synthesis parts, the 6 riboregulators, into pSB1C3.
- Week 7 (22-28 July)
22/07
Assembling the riboregulators in pSB1C3 … again…
August 2013
- Week 8 (29-4 August)
30/7
Change of vector for the six riboswitches : from the industrial one to PSB1C3 after Xba1/Spe1 restriction (only sites available on the synthesis).
PCR to extract the recombinase PhiC31 (after mutation to delete the EcoR1 site).
31/07
0,8% gels to confirm:
- mutation and extraction of PCR products : some correct inserts
- riboswitches transfer in PSB1C3 : R0, R1 OK
Cloning:
- PhiC31 in PSB1C3 carrying a terminator
- PhiC31 + terminator in PSB1K3 with 3A method
- PhiC31 in PSB1C3
- R0 + RFP
- R1 + RFP
01/08
2mL culturs then minipreps to confirm clonings.
02/08
Clonings:
- Transfer of R2, R3, R5 in PSB1C3
- RFP in PSB1A2
- Week 9 (5-11 August)
05/08
Minipreps then 0,8% gel : RFP in PSBA2 OK, others failed.
06/08
Stock of pSB1C3 Xba1/Spe1 digested to continue riboswitches transfer : gel extraction.
08/08
Still trying to clone : R2, R3, R4 and R5 in PSB1C3
09/08
No clone for R3 and R5.
Minipreps and 0,8% gel for others:
- correct inserts for R2 (450 bp) and R4 (600bp) after E/P restriction.
- correct length for Phic31, but not the right sequence according to more restrictions.
- Week 10 (12-18 August)
12/06
Cloning:
- Transfer of R3, R5 in PSB1C3
New PCR to extract mutant PhiC31
13/08
No clone obtained. May be a problem with competent cells. New transformation of ligation of 12th august.
14/08
New try to transfer R3, R5 in pSB1C3.
Gel extraction of mutant PhiC31 and transfer into strata clone.
15/08
Cloning:
- R3 in pSB1C3
- Tp901 in pSB1C3
- Bxb1 in Psb1C3
- Week 11 (19-25 August)
19/08
0,8% gel:
- no insert for tp901 cloning
- correct inserts for bxb1, R2 and R4 after E/P restriction
20/08
Gel extraction of riboswitches : R0, R1, R2, R4
Assembly of promoter pLac thanks to two homolog oligonucleotides.
Cloning to change promoter in the middle of riboswitches R0, R1, R2, R4. pLac replaces the original one.
Restriction of riboswitches and pLac with BamH1/Cla1 and gel extraction before ligation.
21/08
2% gel to confirm promoter changes:
- E/P restriction : correct size for R1pLac but others failed
- confirmation by EcoR1, BamH1 and EcoR1/BamH1 restriction : failed.
No clone with a correct change of promoter obtained.
New range of cloning with pLac into riboswitches.
22/08
Minipreps and 0.8% gel : clonings failed.
- Week 12 (26-31 August)
26/08
After some research, riboswitches on the synthesis have EcoR1/Xba1 on one side and Spe1 on the other side. Restriction with EcoR1/Spe1 will be more efficient than Xba1/Spe1 because these second couple of sites are compatible. Decision to start over with this new information.
New start : gel extraction of R0, R1, R2 and R4 from the commercial plasmid digested by EcoR1/Spe1. Then cloning into Psb1C3 (R3 and R5 have been leave out because no good results were ever obtained with them and time begin to lack. We have to focus on what is possible).
September 2013
- Week 13 (1-8 September)
02/09
1,5 % gel to confirm clonings after restriction by EcoRI, PstI, EcoRI/PstI, BamH1:
- R1pLac-1 : confirmed !
- R1pLac-2 : confirmed !
- R4 in pSB1C3 : failed
Clonings:
- R1pLac 1 + RFP
- R1pLac 2 + RFP
New gel extraction to change promoter:
- R4 digested BamHI/ClaI
- R2 digested BamHI/ClaI
To transfer into pSB1C3: R0 digested EcoRI/SpeI
03/09
Minipreps and 0,8%gel after EcoRI/PstI digestion : R1pLac 1 + RFP and R1plac 2 + RFP CONFIRMED !
Clonings:
- R0 into pSB1C3
- R2 + pLac
- R4 + pLac
04/09
Confirmation of R1plac+RFP clonings with a 1,5% gel after following digestions : EcoRI, PstI, BamHI → OK!
05/09
Clonings : strong promoter (C) + tetR (C)
06/09
Minipreps and 0,8%gel after EcoRI/PstI digestion : failed
- Week 14 (9-15 September)
09/09
New try to clone strong promoter strong rbs (C) and tetR (on pSB1A2 this time)
10/09
Minipreps and 0,8%gel after EcoRI/PstI digestion : successfull cloning
11/09
Clonings : - Final construct : R1pLac RFP (C) + strong promoter strong rbs tet R (C)
- Transfer of R1Plac RFP (C) into pSB1A2
12/09
Minipreps and 0,8%gel after EcoRI/PstI digestion :
- Final construct : failed
- Transfer : OK
13/09
Clonings : - Final construct : R1plac RFP (A) + strong promoter strong rbs tet R (C)
- Construct to test a recombinase : R1pLac (C) + Bxb1 (A)
- Week 15 (16-22 September)
16/09
Minipreps and 0,8%gel after EcoRI/PstI digestion : - Final construct : correct size -> Sequencing
- Recombinase : correct insert -> Sequencing
17/09
We realized that our RFP doesn't have a terminator at the end, so we have to make the cloning in the opposite order.
Clonings : Final construct : strong promoter strong rbs tet R (C) + R1plac RFP (A)
18/09
Sequencing results : - Final construct : Failed
- Recombinase : OK
Minipreps and 0,8%gel after EcoRI/PstI digestion : - Final construct : correct size
EcoRI/BamHI digestion : failed. 19/09
Clonings : - Final construct : R1plac RFP (A) + strong promoter strong rbs tet R (C)
- Construct to test a recombinase : strong promoter strong rbs + R1pLac Bxb1
20/09
Minipreps and 0,8%gel after EcoRI/PstI digestion : Two clonings failed.
 "
"


