Team:Paris Saclay/Notebook/August/13
From 2013.igem.org
(Created page with "{{Team:Paris_Saclay/incl_debut_generique}} ='''Notebook : August 8'''= =='''Lab work'''== ==='''A - Aerobic/Anaerobic regulation system'''=== ===='''Obtaining the PSB3K3 back...") |
(→2 - Electrophoresis to check the digestion of BBa_J04450 by EcoRI/PstI) |
||
| (97 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Paris_Saclay/incl_debut_generique}} | {{Team:Paris_Saclay/incl_debut_generique}} | ||
| - | ='''Notebook : August | + | ='''Notebook : August 13'''= |
=='''Lab work'''== | =='''Lab work'''== | ||
| Line 7: | Line 7: | ||
==='''A - Aerobic/Anaerobic regulation system'''=== | ==='''A - Aerobic/Anaerobic regulation system'''=== | ||
| - | ====''' | + | ===='''Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== |
| - | ===='''1 - Electrophoresis | + | ===='''1 - Electrophoresis to check the digestion of BBa_K1155000 by SpeI/PstI'''==== |
Nadia | Nadia | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |[[]] | + | | style="width:350px;border:1px solid black;" |[[File:Psgel11308.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| - | *Well 1 : 6µL DNA Ladder | + | * Well 1 : 6µL DNA Ladder |
| - | *Well 2 : 5µL | + | * Well 2 : 5µL BBa_K1155000 digested by SpeI/PstI +1µl of 6X loading dye |
| - | *Gel : 0.8% | + | * Gel : 0.8% |
|} | |} | ||
Expected sizes : | Expected sizes : | ||
| - | * | + | * Pndh* : 111bp |
| - | * | + | * pSB1C3 : 2070bp |
| - | + | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We can't see any band for | + | We can't see any band for BBa_K1155000 digestion. The digestion failed because we used the wrong enzymes. We will do it again with the good ones. |
|} | |} | ||
| - | ==== | + | ===='''2 - Extraction of BBa_K1155004, BBa_1155005, BBa_K1155006 from DH5αstrain'''==== |
| + | |||
| + | XiaoJing | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/extraction|High-copy plasmid extraction]] | ||
| + | |||
| + | ===='''3 - Digestion of BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006 by SpeI and by SpeI/EcoRI'''==== | ||
Anaïs, Nadia | Anaïs, Nadia | ||
| + | Used quantities : | ||
| + | * BBa_K1155000 : | ||
| + | ** Buffer FD : 5µL | ||
| + | ** H2O : 39µL | ||
| + | ** DNA : 5µL | ||
| + | ** SpeI FD : 1µL | ||
| - | + | * BBa_K1155000 : | |
| + | ** Buffer FD : 5µL | ||
| + | ** H2O : 38µL | ||
| + | ** DNA : 5µL | ||
| + | ** SpeI FD : 1µL | ||
| + | **EcoRI FD : 1µL | ||
| - | + | * BBa_K1155004, BBa_K1155005, BBa_K1155006 : | |
| + | ** Buffer FD : 2µL | ||
| + | ** H2O : 7µL | ||
| + | ** DNA : 10µL | ||
| + | ** SpeI FD : 1µL | ||
| - | + | * BBa_K1155004, BBa_K1155005, BBa_K1155006 : | |
| + | ** Buffer FD : 2µL | ||
| + | ** H2O : 6µL | ||
| + | ** DNA : 10µL | ||
| + | ** SpeI FD : 1µL | ||
| + | ** EcoRI FD : 1µL | ||
| - | + | We incubat the digestion at 37°C during 10 minutes. | |
| - | ====''' | + | ====4 - '''Electrophoresis to check the digestion of BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006 by SpeI and by SpeI/EcoRI'''==== |
| - | + | Anaïs, Nadia | |
| - | Anaïs | + | |
| - | * | + | {| |
| - | ** | + | | style="width:350px;border:1px solid black;" |[[File:Psgel21308.jpg]] |
| - | ** | + | | style="width:350px;border:1px solid black;vertical-align:top;" | |
| + | * Well 1 : 6µL DNA Ladder | ||
| + | * Well 2 : 5µL BBa_K1155000 digested by SpeI + 1µl of 6X loading dye | ||
| + | * Well 3 : 5µL BBa_K1155000 +1µl of 6X loading dye | ||
| + | * Well 4 : 5µL BBa_K1155000 digested by EcoRI/Spe I + 1µl of 6X loading dye | ||
| + | * Gel : 0.8% | ||
| + | |} | ||
| - | * | + | Expected sizes : |
| + | * Pndh* : 111bp | ||
| + | * pSB1C3 : 2070 kb | ||
| - | + | {| | |
| - | + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | |
| - | + | We lost all our digestion product so we will do it again. | |
| - | + | |} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | {| | |
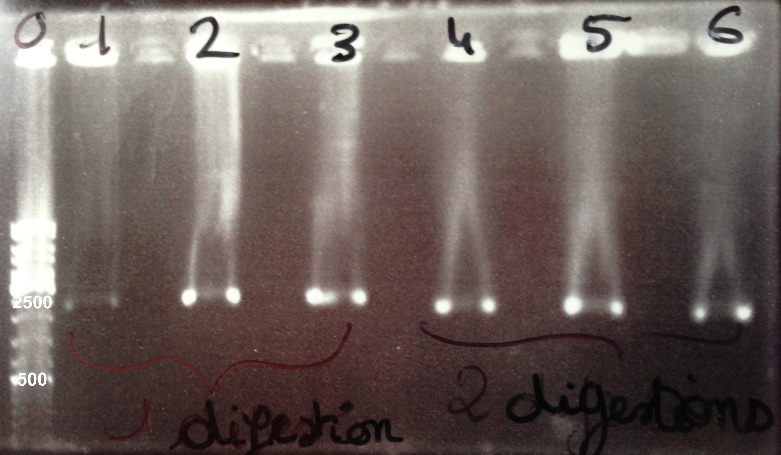
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel31308.jpg|500px]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 0 : 6µL DNA Ladder | ||
| + | * Well 1 : 5µL BBa_K1155006 digested by SpeI + 1µl of 6X loading dye | ||
| + | * Well 2 : 5µL BBa_K1155005 digested by SpeI + 1µl of 6X loading dye | ||
| + | * Well 3 : 5µL BBa_K1155004 digested by SpeI + 1µl of 6X loading dye | ||
| + | * Well 4 : 5µL BBa_K1155006 digested by EcoRI/SpeI + 1µl of 6X loading dye | ||
| + | * Well 5 : 5µL BBa_K1155005 digested by EcoRI/SpeI + 1µl of 6X loading dye | ||
| + | * Well 6 : 5µL BBa_K1155004 digested by EcoRI/SpeI + 1µl of 6X loading dye | ||
| + | * Gel : 0.8% | ||
| + | |} | ||
| - | + | Expected sizes : | |
| + | * NarK, Nar G, NirB : 200kb | ||
| + | * pSB1C3 : 2070bp | ||
| + | * NarK in pSB1C3, NarG in pSB1C3, NirB in pSB1C3 : 2270bp | ||
| - | + | {| | |
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We lost all our double digestion product so we will do it again. We obtain BBa_K1155004, BBa_K1155005, BBa_K1155006 digested by SpeI fragments at the right size. We can purify it. | ||
| + | |} | ||
| - | ===== | + | ===='''5 - Liquid culture of MG1655Z1 Δfnr::Km'''==== |
| - | Anaïs, | + | |
| + | XiaoJing | ||
| + | |||
| + | We picked up one colony in 5mL of LB and 5µL of kanamycine. We do it twice. | ||
| + | |||
| + | We incubated our culture at 37°C with agigation at 150rpm. | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtain fragments at the right size. | ||
| + | |} | ||
| + | |||
| + | ===='''Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in pSB3K3'''==== | ||
| + | |||
| + | ===='''1 - Digestion of BBa_J04450 by EcoRI/PstI'''==== | ||
| + | |||
| + | Anaïs | ||
| + | |||
| + | Used quantities : | ||
| + | |||
| + | * Buffer FD: 2µL | ||
| + | * H2O : 6µL | ||
| + | * DNA : 10µL | ||
| + | * EcoRI FD : 1µL | ||
| + | * PstI FD : 1µL | ||
| + | |||
| + | We incubated the digestion at 37°C during 10 minutes. | ||
| + | |||
| + | ===='''2 - Electrophoresis to check the digestion of BBa_J04450 by EcoRI/PstI'''==== | ||
| + | |||
| + | Anaïs, Nadia | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" | [[File: | + | | style="width:350px;border:1px solid black;" |[[File:Psgel41308.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| - | *6µL DNA Ladder | + | * Well 4 : 6µL DNA Ladder |
| - | * | + | * Well 7 : 5µL of BBa_J04450 digested by EcoRI/PstI + 1µL of 6X loading dye |
| - | *Gel : 0.8% | + | * Gel : 0.8% |
|} | |} | ||
| - | Expected | + | Expected sizes : |
| + | * pSB3K3 : 2750bp | ||
| + | * GFP : 1069bp | ||
| - | + | {| | |
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtained a fragment at the right size. | ||
| + | |} | ||
| + | |||
| + | ==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== | ||
| + | |||
| + | ===='''Objective : obtaining FNR and BphR2 proteins (Gibson assembly)'''==== | ||
| + | |||
| + | ===='''1 - PCR of FRN Part I, FNR Part II, BphR2 Part I'''==== | ||
| + | |||
| + | Anaïs | ||
| + | |||
| + | Used quantities : | ||
| + | * Bphr2 Part I : | ||
| + | ** Oligo 54F : 1µL | ||
| + | ** Oligo 55R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA of ''Pseudomonas pseudoalcaligenes'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| + | |||
| + | * FNR Part I : | ||
| + | ** Oligo 59F : 1µL | ||
| + | ** Oligo 60R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA ''Escherichia coli'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| + | |||
| + | * FNR Part II : | ||
| + | ** Oligo 61F : 1µL | ||
| + | ** Oligo 62R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA ''Escherichia coli'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| + | |||
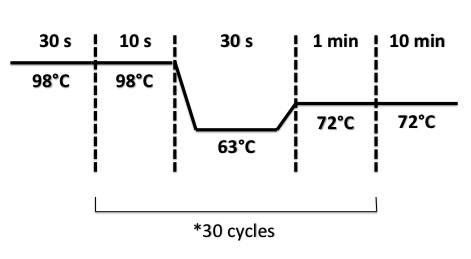
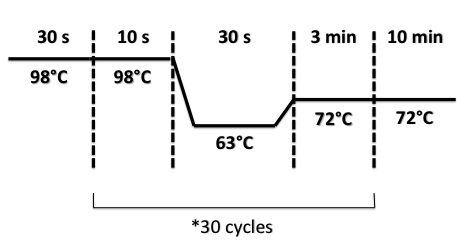
| + | PCR Program : | ||
| + | |||
| + | * BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I : | ||
| + | |||
| + | [[File:PsPCRBphR23007.jpg|400px]] | ||
| + | |||
| + | * FNR Part I, FNR Part II, RBS-FNR Part I : | ||
| + | |||
| + | [[File:PsPCRFNR3007.jpg|400px]] | ||
| + | |||
| + | ===='''2 - Electrophoresis of PCR products : FRN Part I, FNR Part II, BphR2 Part I'''==== | ||
| - | |||
Damir | Damir | ||
| - | + | {| | |
| - | + | | style="width:350px;border:1px solid black;" |[[File:Psgel51308.jpg]] | |
| - | * | + | | style="width:350px;border:1px solid black;vertical-align:top;" | |
| - | * | + | * Well 1 : 5µL FNR Part II + 1µl of 6X loading dye |
| - | * | + | * Well 2 : 5µL BphR2 Part I + 1µl of 6X loading dye |
| - | * | + | * Well 3 : 5µL FNR Part I + 1µl of 6X loading dye |
| - | * | + | * Well 4 : 6µL DNA Ladder |
| + | * Gel : 1% | ||
| + | |} | ||
| - | + | {| | |
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel61308.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 1 : 5µL FNR Part II + 1µl of 6X loading dye | ||
| + | * Well 2 : 5µL BphR2 Part I + 1µl of 6X loading dye | ||
| + | * Well 3 : 5µL FNR Part I + 1µl of 6X loading dye | ||
| + | * Well 4 : 6µL DNA Ladder | ||
| + | * Gel : 1% | ||
| + | |} | ||
| - | + | Expected sizes : | |
| + | * FNR Part I : 597 kb | ||
| + | * FNR Part II : 200 kb | ||
| + | * BphR2 Part I : 178 kb | ||
| - | + | {| | |
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | It's impossible to read the first gel. We do it again. | ||
| + | In the second gel, we obtain FNR Part I and FNR Part II fragments at the right size. We can purify it. We also obtain BphR2 Part I frangment at the right size but it was mix with other DNA frangments. We will try to make a gel purification of it. | ||
| + | |} | ||
| + | ===='''3 - Electrophoresis of PCR product : BphR2 Part I'''==== | ||
| + | Anaïs, Damir, Nadia | ||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel71308.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 1 : 6µL DNA Ladder | ||
| + | * Well 2 : 40µL of BphR2 Part I + 8µL of 6X loading dye | ||
| + | * Gel : 1% | ||
| + | |} | ||
| + | |||
| + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | Even if the two lightest stripes are overlapping, aims to do a second gel purification with these two stripes, we did the gel purification . After argumentation, we decided to do the PCR of BphR2 Part I again thanks to new PCR program and new quantities. | ||
| + | |} | ||
| + | |||
| + | ===='''4 - PCR of BphR2 Part I'''==== | ||
| + | |||
| + | Anaïs | ||
| + | |||
| + | Used quantities : | ||
| + | * Oligo 54F : 1µL | ||
| + | * Oligo 55R : 1µL | ||
| + | * DNA : 2µL | ||
| + | * Buffer Phusion : 10µL | ||
| + | * dNTP : 1µL | ||
| + | * Phusion : 1µL | ||
| + | * H2O : 33.5µL | ||
| + | |||
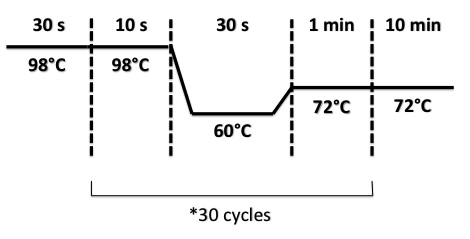
| + | PCR Program : | ||
| + | |||
| + | [[File:PsPCRBphR2PI1308.jpg|400px]] | ||
| + | |||
| + | '''5 - Gel purification of PCR product : FNR Part I, FNR Part II, RBS-FNR Part I, BphR2 Part II, | ||
| + | '''RBS-BphR2 Part I'''''' | ||
| + | |||
| + | XiaoJing | ||
| + | |||
| + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] | ||
| + | |||
| + | Nanodrop : | ||
| + | * FNR Part I : 152.7ng/µL | ||
| + | * FNR Part II : 137.2ng/µL | ||
| + | * RBS-FNR Part I : 153.7ng/µL | ||
| + | * BphR2 Part II : 129.9ng/µL | ||
| + | * RBS-BphR2 Part I : 158.1ng/µL | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | The purification was good. We will do the Gibson assembly. | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| border="1" align="center" | ||
| + | |[[Team:Paris Saclay/Notebook/August/12|<big>Previous day</big>]] | ||
| + | |||
| + | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| + | |||
| + | |[[Team:Paris Saclay/Notebook/August/14|<big>Next day</big>]] | ||
| + | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 01:28, 5 October 2013
Notebook : August 13
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - Electrophoresis to check the digestion of BBa_K1155000 by SpeI/PstI
Nadia
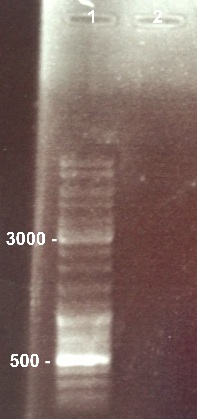
|
|
Expected sizes :
- Pndh* : 111bp
- pSB1C3 : 2070bp
|
We can't see any band for BBa_K1155000 digestion. The digestion failed because we used the wrong enzymes. We will do it again with the good ones. |
2 - Extraction of BBa_K1155004, BBa_1155005, BBa_K1155006 from DH5αstrain
XiaoJing
Protocol : High-copy plasmid extraction
3 - Digestion of BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006 by SpeI and by SpeI/EcoRI
Anaïs, Nadia
Used quantities :
- BBa_K1155000 :
- Buffer FD : 5µL
- H2O : 39µL
- DNA : 5µL
- SpeI FD : 1µL
- BBa_K1155000 :
- Buffer FD : 5µL
- H2O : 38µL
- DNA : 5µL
- SpeI FD : 1µL
- EcoRI FD : 1µL
- BBa_K1155004, BBa_K1155005, BBa_K1155006 :
- Buffer FD : 2µL
- H2O : 7µL
- DNA : 10µL
- SpeI FD : 1µL
- BBa_K1155004, BBa_K1155005, BBa_K1155006 :
- Buffer FD : 2µL
- H2O : 6µL
- DNA : 10µL
- SpeI FD : 1µL
- EcoRI FD : 1µL
We incubat the digestion at 37°C during 10 minutes.
4 - Electrophoresis to check the digestion of BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006 by SpeI and by SpeI/EcoRI
Anaïs, Nadia
Expected sizes :
- Pndh* : 111bp
- pSB1C3 : 2070 kb
|
We lost all our digestion product so we will do it again. |
Expected sizes :
- NarK, Nar G, NirB : 200kb
- pSB1C3 : 2070bp
- NarK in pSB1C3, NarG in pSB1C3, NirB in pSB1C3 : 2270bp
|
We lost all our double digestion product so we will do it again. We obtain BBa_K1155004, BBa_K1155005, BBa_K1155006 digested by SpeI fragments at the right size. We can purify it. |
5 - Liquid culture of MG1655Z1 Δfnr::Km
XiaoJing
We picked up one colony in 5mL of LB and 5µL of kanamycine. We do it twice.
We incubated our culture at 37°C with agigation at 150rpm.
|
We obtain fragments at the right size. |
Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in pSB3K3
1 - Digestion of BBa_J04450 by EcoRI/PstI
Anaïs
Used quantities :
- Buffer FD: 2µL
- H2O : 6µL
- DNA : 10µL
- EcoRI FD : 1µL
- PstI FD : 1µL
We incubated the digestion at 37°C during 10 minutes.
2 - Electrophoresis to check the digestion of BBa_J04450 by EcoRI/PstI
Anaïs, Nadia

|
|
Expected sizes :
- pSB3K3 : 2750bp
- GFP : 1069bp
|
We obtained a fragment at the right size. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins (Gibson assembly)
1 - PCR of FRN Part I, FNR Part II, BphR2 Part I
Anaïs
Used quantities :
- Bphr2 Part I :
- Oligo 54F : 1µL
- Oligo 55R : 1µL
- Buffer Phusion : 10µL
- DNA of Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- FNR Part I :
- Oligo 59F : 1µL
- Oligo 60R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- FNR Part II :
- Oligo 61F : 1µL
- Oligo 62R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
PCR Program :
- BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I :
- FNR Part I, FNR Part II, RBS-FNR Part I :
2 - Electrophoresis of PCR products : FRN Part I, FNR Part II, BphR2 Part I
Damir

|
|
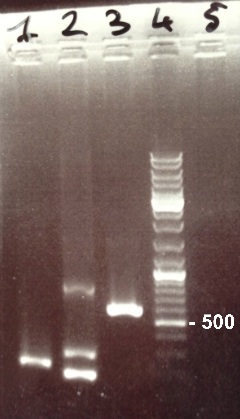
|
|
Expected sizes :
- FNR Part I : 597 kb
- FNR Part II : 200 kb
- BphR2 Part I : 178 kb
|
It's impossible to read the first gel. We do it again. In the second gel, we obtain FNR Part I and FNR Part II fragments at the right size. We can purify it. We also obtain BphR2 Part I frangment at the right size but it was mix with other DNA frangments. We will try to make a gel purification of it. |
3 - Electrophoresis of PCR product : BphR2 Part I
Anaïs, Damir, Nadia

|
|
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
|
Even if the two lightest stripes are overlapping, aims to do a second gel purification with these two stripes, we did the gel purification . After argumentation, we decided to do the PCR of BphR2 Part I again thanks to new PCR program and new quantities. |
4 - PCR of BphR2 Part I
Anaïs
Used quantities :
- Oligo 54F : 1µL
- Oligo 55R : 1µL
- DNA : 2µL
- Buffer Phusion : 10µL
- dNTP : 1µL
- Phusion : 1µL
- H2O : 33.5µL
PCR Program :
5 - Gel purification of PCR product : FNR Part I, FNR Part II, RBS-FNR Part I, BphR2 Part II, RBS-BphR2 Part I'
XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- FNR Part I : 152.7ng/µL
- FNR Part II : 137.2ng/µL
- RBS-FNR Part I : 153.7ng/µL
- BphR2 Part II : 129.9ng/µL
- RBS-BphR2 Part I : 158.1ng/µL
|
The purification was good. We will do the Gibson assembly. |
| Previous day | Back to calendar | Next day |
 "
"