Team:Paris Saclay/Notebook/August/21
From 2013.igem.org
(Difference between revisions)
| Line 66: | Line 66: | ||
[[File:PsPCRAmilCP2108.jpg|400px]] | [[File:PsPCRAmilCP2108.jpg|400px]] | ||
| - | + | ((((((((====3 - Culture ==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ====3 - Culture ==== | + | |
Nguyen | Nguyen | ||
| Line 84: | Line 72: | ||
*strain MG1665 Δ fnr:km clone1 in 5ml LB with kanamicine | *strain MG1665 Δ fnr:km clone1 in 5ml LB with kanamicine | ||
| - | Incubate at 37°C over night with shake 180 rpm. | + | Incubate at 37°C over night with shake 180 rpm.)))))))))))))))))))) |
===='''Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in PSB3K3'''==== | ===='''Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in PSB3K3'''==== | ||
| Line 110: | Line 98: | ||
| - | ==='''A - Aerobic/Anaerobic regulation system / B - PCB | + | ==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== |
| - | + | ||
| - | + | ||
| - | + | ===='''1 - Gibson assembly.'''==== | |
| - | + | ||
| - | + | ||
| + | Xiaojing | ||
| + | Used quantities : | ||
| - | + | * RBS-BphR2 : | |
| - | + | ** PSB1C3 : 3µL | |
| - | + | ** BphR2 Part I : 1µL | |
| - | + | ** BphR2 Part II : 1µL | |
| - | * | + | ** Gibson mix : 15µL ??????????????? |
| - | * | + | |
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | * FNR : | ||
| + | ** PSB1C3 : 3µL | ||
| + | ** FNR Part I : 1µL | ||
| + | ** FNR Part II : 1µL | ||
| + | ** Gisbon mix : 15µL ??????????????? | ||
| + | * RBS-FNR : | ||
| + | ** PSB1C3 : 3µL | ||
| + | ** RBS-FNR Part I : 1µL | ||
| + | ** FNR Part II : 1µL | ||
| + | ** Gibson mix : 15µL ???????????? | ||
| + | We let these mix at 50°C during 1h. | ||
| + | ====2 - Transformation of RBS-BphR2, FNR, RBS-FNR in DH5α==== | ||
| + | XiaoJing | ||
| + | Protocol : [[Team:Paris_Saclay/Protocols/bacterial transformation|Bacterial transformation]] | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Revision as of 15:45, 17 September 2013
Contents
|
Notebook : August 21
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
1 - Colony PCR of NarK, NarG or NirB with RBS-LacZ-Term or RBS-AmilCP-Term in PSB1C3 in DH5α
Damir, Xiaojing
Colonies repiquée dans 10µL d'eau. We do 8 PCR for each ligation.
Used quantities :
- Mix A :
- Buffer Dream Taq : 250µL
- dNTP : 50µL
- Oligo 44 : 50µL
- Dream Taq : 20µL
- H2O : 1.88mL
- NirB with RBS-LacZ-Term in PSB1C3 :
- 45µL of Mix A+1µL of oligo 45+2µL of DNA
- NirB with RBS-Amil CP-Term in PSB1C3 :
- 45µL of Mix A+1µL of oligo 45 +2µL of DNA
- NirB with RBS-LacZ-Term in PSB1C3 :
- NarG with RBS-LacZ-Term in PSB1C3 :
- 45µL of Mix A+1µL of oligo 41+2µL of DNA
- NarG with RBS-Amil CP-Term in PSB1C3 :
- 45µL of Mix A+1µL of oligo 41 +2µL of DNA
- NarG with RBS-LacZ-Term in PSB1C3 :
- NarK with RBS-LacZ-Term in PSB1C3 :
- 45µL of Mix A+1µL of oligo 47+2µL of DNA
- NarK with RBS-Amil CP-Term in PSB1C3 :
- 45µL of Mix A+1µL of oligo 47 +2µL of DNA
- NarK with RBS-LacZ-Term in PSB1C3 :
- Mix B :
- Mix A : 1.125mL
- Oligo 43 : 25µL
- NirB with RBS-LacZ-Term in PSB1C3 :
- 23µL of Mix B+2µL of DNA
- NirB with RBS-Amil CP-Term in PSB1C3 :
- 23µL of Mix B+2µL of DNA
- NirB with RBS-LacZ-Term in PSB1C3 :
- NarG with RBS-LacZ-Term in PSB1C3 :
- 23µL of Mix B+2µL of DNA
- NarG with RBS-Amil CP-Term in PSB1C3 :
- 23µL of Mix B+2µL of DNA
- NarG with RBS-LacZ-Term in PSB1C3 :
- NarK with RBS-LacZ-Term in PSB1C3 :
- 23µL of Mix B+2µL of DNA
- NarK with RBS-Amil CP-Term in PSB1C3 :
- 23µL of Mix B+2µL of DNA
- NarK with RBS-LacZ-Term in PSB1C3 :
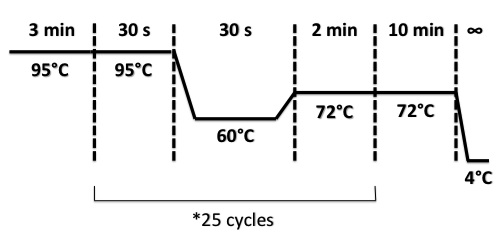
PCR Program for NirB with RBS-LacZ-Term in PSB1C3, NarG with RBS-LacZ-Term in PSB1C3, NarK with RBS-LacZ-Term in PSB1C3 :
PCR Program for NirB with RBS-Amil CP-Term in PSB1C3, NarG with RBS-Amil CP-Term in PSB1C3, NarK with RBS-Amil CP-Term in PSB1C3 :
((((((((====3 - Culture ==== Nguyen
- BBa_K115500 strains (FNR repressor)in 5ml LB with Chlorenphenicol
- strain MG1665 Δ fnr:km clone1 in 5ml LB with kanamicine
Incubate at 37°C over night with shake 180 rpm.))))))))))))))))))))
Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in PSB3K3
1 - Electrophoresis of the digestion of Bba_J04450 by EcoRI/PstI
XiaoJing
| [[]] |
|
Expected sizes :
- PSB3K3 : 2750 bp
- GFP : ... NON VISIBLE SUR LE GEL ???????
|
We obtain a fragment at the right size. We can purify it. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
1 - Gibson assembly.
Xiaojing
Used quantities :
- RBS-BphR2 :
- PSB1C3 : 3µL
- BphR2 Part I : 1µL
- BphR2 Part II : 1µL
- Gibson mix : 15µL ???????????????
- FNR :
- PSB1C3 : 3µL
- FNR Part I : 1µL
- FNR Part II : 1µL
- Gisbon mix : 15µL ???????????????
- RBS-FNR :
- PSB1C3 : 3µL
- RBS-FNR Part I : 1µL
- FNR Part II : 1µL
- Gibson mix : 15µL ????????????
We let these mix at 50°C during 1h.
2 - Transformation of RBS-BphR2, FNR, RBS-FNR in DH5α
XiaoJing
Protocol : Bacterial transformation
 "
"