Team:Paris Saclay/Notebook/August/27
From 2013.igem.org
(Difference between revisions)
| Line 138: | Line 138: | ||
* Pfnr with RBS-LacZ-Term in PSB1C3 : | * Pfnr with RBS-LacZ-Term in PSB1C3 : | ||
* Pfnr with RBS-Amil CP-Term in PSB1C3 : | * Pfnr with RBS-Amil CP-Term in PSB1C3 : | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtain fragments at the right size for NirB with RBS-Amil CP-Term in PSB1C3 in well 12, Pfnr with RBS-LacZ-Term in PSB1C3 in well 14, 15, 18 and 19 and Pfnr with RBS-Amil CP-Term in PSB1C3 in well 20, 22 and 25. Nevertheless, electrophoresis shows that these colonies weren't pure. | ||
| + | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Revision as of 18:19, 21 September 2013
Contents |
Notebook : August 27
summary
- We got colonies after the transformation of E.coli with the ligation mix "PnirB_PSB1C3 and RBS_LacZ_Term"
"PnirB_PSB1C3 plus RBS_AmilCP+Term" "Pndh*_PSB1C3 plus RBS_LacZ+Term" "Pndh*_PSB1C3 plus RBS_AmilCP+Term" so we tested for correct ligation with PCR on colonies
- We did not get any colonies for the Gibson assembly so we analyzed by gel electrophoresis the parts used for the Gibson assembly to verify their sizes and concentrations.
- Digestion DnpI purification of the the PSB1C3 clean for Gibson and electrophoresis of it .
A - Aerobic/Anaerobic regulation system / B - PCB sensing system
1 -Gel electrophoresis of parts of Gibson assembly.
| [[]] |
|
Now we do a Digestion Dnp1 purification of the the PSB1C3 clean for Gibson.
| PSB1C3 clean for Gibson | 17µl |
| enzyme Dnp1 | 1µl |
| buffer | 2µl |
| total | 20µl |
- Incubate at 37°C for 1h30mins.
2 - Gel purification of the PSB1C3 Clean for Gibson
2.1 - Migration of 20µl PSB1C3 Clean for Gibson digested by Dnp1 '
| [[]] |
|
| Previous week | Back to calendar | Next day |
Notebook : August 5
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155004, Bba_K1155005, Bba_K1155006
1 - Colony PCR of ligation Pfnr or NirB with RBS-LacZ-Term or RBS-Amil CP-Term in PSB1C3
XiaoJing
|
Transformation of 08/26/13 works. We will do a PCR Colony. |
COLONIES PIQUEES DANS 10µL d'eau par Tube !!!!!!!!!!!!!
Used quantities :
- DNA : 2µL
- Mix : (it was divided in 6 tubes for each promotor with 23µL of mix in each tube)
- Oligo 44 : 17.5µL
- Oligo 43 : 17.5µL
- Buffer Dream Taq : 87.5µL
- dNTP : 17.5µL
- Dream Taq : 7µL
- H2O : 591µL
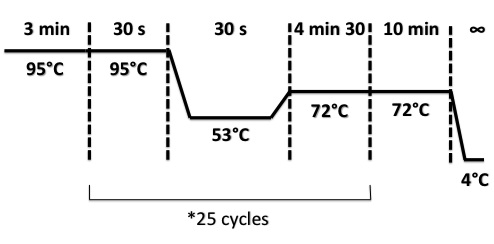
PCR Program :
2 - Gel electrophoresis of the colony PCR products : Pfnr or NirB with RBS-LacZ-Term or RBS-Amil CP-Term in PSB1C3
XiaoJing
| [[]] |
|
Expected size : 3583bp ????
- NirB with RBS-LacZ-Term in PSB1C3 :
- NirB with RBS-Amil CP-Term in PSB1C3 :
- Pfnr with RBS-LacZ-Term in PSB1C3 :
- Pfnr with RBS-Amil CP-Term in PSB1C3 :
|
We obtain fragments at the right size for NirB with RBS-Amil CP-Term in PSB1C3 in well 12, Pfnr with RBS-LacZ-Term in PSB1C3 in well 14, 15, 18 and 19 and Pfnr with RBS-Amil CP-Term in PSB1C3 in well 20, 22 and 25. Nevertheless, electrophoresis shows that these colonies weren't pure. |
 "
"