Team:Paris Saclay/Notebook/August/9
From 2013.igem.org
(Difference between revisions)
| Line 18: | Line 18: | ||
| style="width:350px;border:1px solid black;" | [[File:Ps908transduction.jpg|350px]] | | style="width:350px;border:1px solid black;" | [[File:Ps908transduction.jpg|350px]] | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
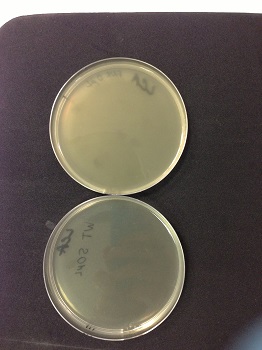
| - | * | + | '''picture: lysed cells comparison.''' |
| - | * | + | * 0µl phage(control): the petri dish is cloudy, bacteria are not lysed |
| - | * | + | * 50µl phage: the petri dish is clear, bacteria are lysed by phages. |
| + | *We used a wild type strain to keep a stock and a Δ ''fnr E. coli'' strain to obtain phages that might have encapsidated a Δfnr::Km fragment. | ||
|} | |} | ||
Revision as of 11:56, 11 August 2013
Contents |
Notebook : August 9
Lab work
A - Aerobic/Anaerobic regulation system
Obtaining Δ fnr E. coli strain by transduction to test our biobricks
Abdou, Anais, Damir, Nadia, XiaoJing
We do the exprience again because the lysis made on wensday didn't happen. It's probably because the phage strain was too old ( 2001)
Protocol : transduction
Now we can do a gel purification of the highest band which contains the PSB3K3 plasmid.
2.2 - Electroelution of the highest band to extract the PSB3K3 plasmid from the gel
Nadia
Protocol : Electroelution
We let the plasmid precipitate during the night.
Obtaining RBS_LacZ+Term_PSB1C3
1 - Colony PCR on e.coli with RBS_LacZ+Term_PSB1C3 for 25 colonies
Anaïs
- Colony counting :
- Low concentration petri dish : 47 colonies
- High concentration petri dish : 145 colonies
- Picking of 25 colonies
- Preparation of 700µL of Master mix
- H2O : 590µL
- dNTP : 28µL
- VF2 primer : 3.5µL
- VR primer : 3.5µL
- DreamTaq buffer 10x : 70µL
- DreamTaq enzyme : 5µL
Protocol : Colony PCR
PCR Program : [IMAGE]
2 - Gel electrophoresis of the colony PCR products
Anaïs, Damir

|
|
Expected size : 3583bp
Colonies 10, 14, 15 exhibit plasmids with the right length.
 "
"