Team:ZJU-China/Project/GhostSensor/Design
From 2013.igem.org
Ghost Sensor: Design
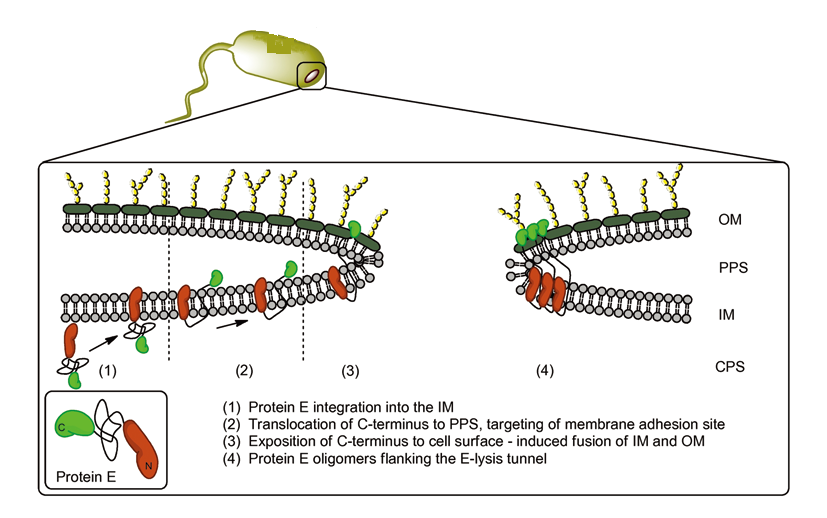
We put Gram-negative bacteria lysis gene protein E under the control of pBAD promoter because of its short inducing time and L-arabinose concentration dependent trait. By making tunnels through inner and outer membrane of E.coli, we solve the conundrum that large molecules cannot enter the cells. At the same time, the periplasmic space remains intact, renders us the chance to utilize it as reaction place.
(Timo Langemann et al., The bacterial ghost platform system Production and applications, Bioengineered Bugs, 2010). The two domains of protein E help to fuse inner- and outer-membrane, keeping the periplasmic space intact.
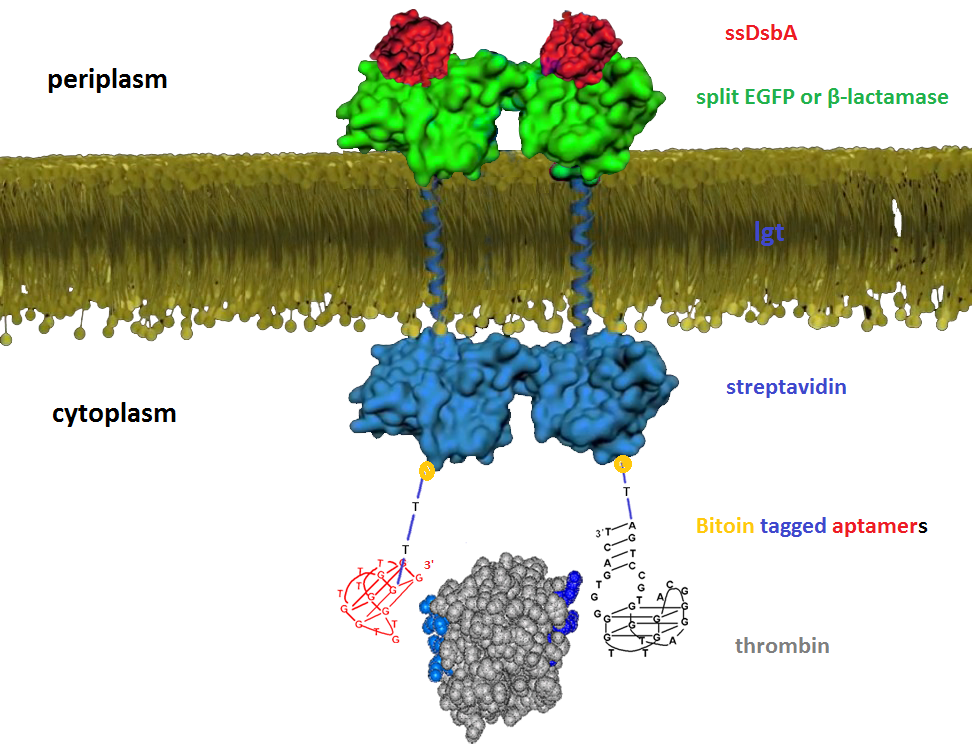
We try to detect thrombin as our proof-of-concept experiment. There have been two well characterized aptamers, one is 15-mer and the other is 29-mer, all show very high and specific affinity to thrombin. We then connected the two aptamers with different length of poly T linkers, namely short and long linkers, to their 5’ end. At last, all aptamers were modified with 5’ biotin in order that they can bind to streptavidin.
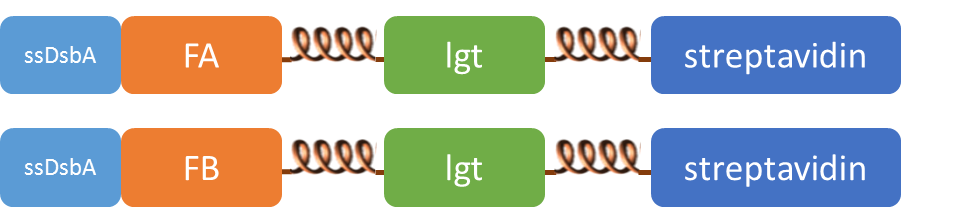
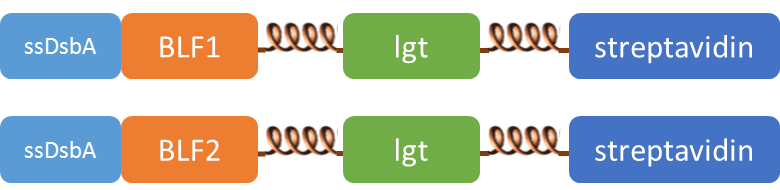
We altogether used two sets of inner-membrane scaffolds, one was based on split EGFP and the other split β-lactamase. Our inner-membrane scaffolds consisted of three fusion parts. The split proteins were linked to the periplasmic ends of scaffolds and streptavidin to the cytoplasmic ends. We chose phosphatidylglycerol:: prolipoprotein diacylglyceryl transferase (Lgt) as our transmembrane protein because its function has been well identified by 2012 SJTU-BioX-Shanghai iGEM team. ssDsbA is the signal recognition particle (SRP)-dependent signaling sequence of DsbA. ssDsbA-tagged proteins are exported to the periplasm through the SRP pathway. With ssDsbA fused to the periplasmic terminal, fusion proteins with Lgt are expected to be anchored onto inner membrane of E.coli.
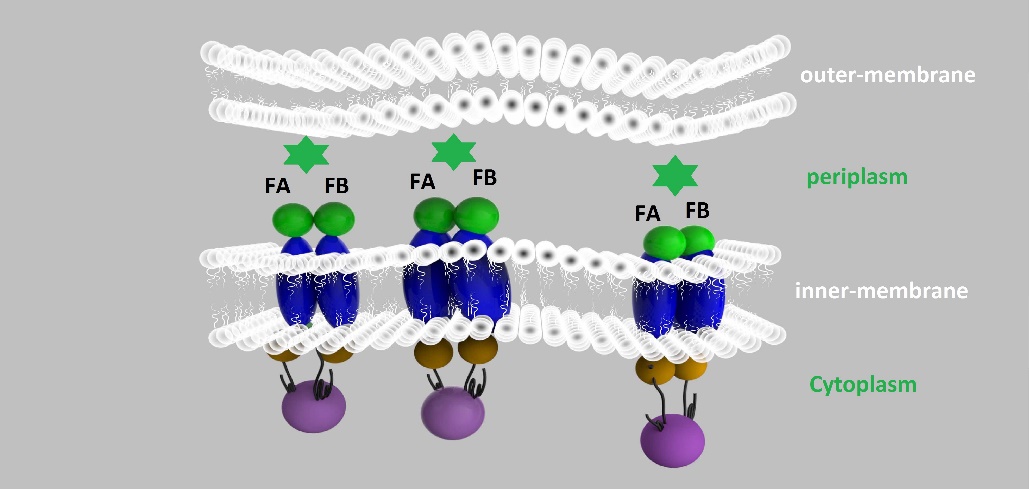
For the first set of membrane scaffolds, two residuals of EGFP (BBa_K738006, BBa_K738007) were fused to the periplasmic ends and streptavidin were fused to the cytoplasmic ends. When 19-mer and 25-mer biotin-modified aptamers are added to the solution and thrombin is present, aptamers act like a bridge linking streptavidin-fused membrane scaffolds and thrombin. In this way, two molecules of membrane scaffolds are pulled together and the two residuals of EGFP will meet each other again to become an integrity part capable of giving green fluorescence. With this design, it is believed that we can detect the existence of any protein through fluorescence.
For the second set of membrane scaffolds, we want to see the results with naked eyes instead of fluorescence to expand its application in everyday life. So we fused two residuals of TEM-1 β-lactamase (BBa_K1054010, BBa_K1054011) to the periplasmic ends. Likewise, when 19-mer and 25-mer biotin-modified aptamers are added to the solution and thrombin is present, aptamers act like a bridge linking streptavidin-fused membrane scaffolds and thrombin. In this way, two molecules of membrane scaffolds are pulled together and the two residuals of β-lactamase will meet each other again to become an integrity part with enzymatic activity.
This activity can be detected by commercial β-lactamase testing strips, which are widely used strips in China to detect the residence of β-lactamase in drinking milk. One mechanism of these strips are based on immune colloidal gold and we chose it because of its high sensitivity and robustness.
 "
"