Team:Bielefeld-Germany/Biosafety/Biosafety System L
From 2013.igem.org
| Line 1: | Line 1: | ||
{{Team:Bielefeld-Germany/Header2}} | {{Team:Bielefeld-Germany/Header2}} | ||
{{Team:Bielefeld-Germany/css/header_cleanup.css}} | {{Team:Bielefeld-Germany/css/header_cleanup.css}} | ||
| + | {{Team:Bielefeld-Germany/css/button.css}} | ||
| + | |||
| + | |||
__NOTOC__ | __NOTOC__ | ||
<html> | <html> | ||
| - | |||
<style> | <style> | ||
| - | . | + | h1{padding:10px 0;padding-left:192px; padding-right:192px; margin-top:70px; } |
| - | + | .toc, #toc{width:255px;font-size:85%;} | |
| - | + | #globalwrapper ul {padding-left:40px; padding-right:40px;} | |
| + | #globalwrapper #rightcol ul {padding-left:0px; padding-right:0px;} | ||
| - | + | h2,h3,h4{clear:both;} | |
| + | #globalwrapper h4{color:#ff6600; padding-left:20px;} | ||
| + | #globalwrapper div.thumb.tleft{margin-left:20px; margin-right:20px; clear:both;} | ||
| - | + | #globalwrapper ul {clear:both;} | |
| - | + | #globalwrapper ul ul{clear:both;} | |
| - | + | #globalwrapper ul{padding-left:60px; padding-right:40px;} | |
| + | #globalwrapper ul ul{padding-left:40px; padding-right:40px;} | ||
| - | + | #globalwrapper p{padding-left:0px; padding-right:40px;} | |
| - | + | #globalwrapper .bigbutton p{padding-left:5px; padding-right:5px; padding-top:2px;} | |
| - | + | ||
| - | + | .bigbutton{width:150px; height:50px; line-height:50px; font-size:1.2em; margin-right:10px; display:table;} | |
| - | + | .bigbutton a{display:block; height:100%;} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | .rightcol{ | |
| + | width:140px; | ||
| + | height:450px; | ||
| + | margin-left:800px; | ||
| + | float:right; | ||
| + | position:fixed; | ||
| + | margin-top:0px; | ||
| + | overflow-y:scroll; | ||
| + | box-shadow:0px 0px 2px 0px grey; | ||
| + | padding:0px 20px; | ||
| + | </style> | ||
| + | </html> | ||
| - | <div | + | <div id=globalwrapper style="padding-left:20px; padding-right:20px"> |
| - | + | <div id="leftcol" style="width:750px; float:left; overflow:auto;"> | |
| - | < | + | |
| - | <div | + | <html> |
| - | + | <h1>Biosafety System Lac of Growth</h1> | |
| - | + | <div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:45px; clear:both;"> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | <div class="bigbutton"> | ||
| + | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Biosafety">Biosafety Overview</a></div> | ||
| + | <div class="bigbutton"> | ||
| + | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System">System Overview</a></div> | ||
| + | <div class="bigbutton"> | ||
| + | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_M#Genetic_Approach">Genetic Approach</a></div> | ||
| + | <div class="bigbutton"> | ||
| + | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_M#Results">Results</a></div> | ||
| - | |||
| - | |||
</div> | </div> | ||
| + | </html> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | ==Overview== | |
| - | + | ||
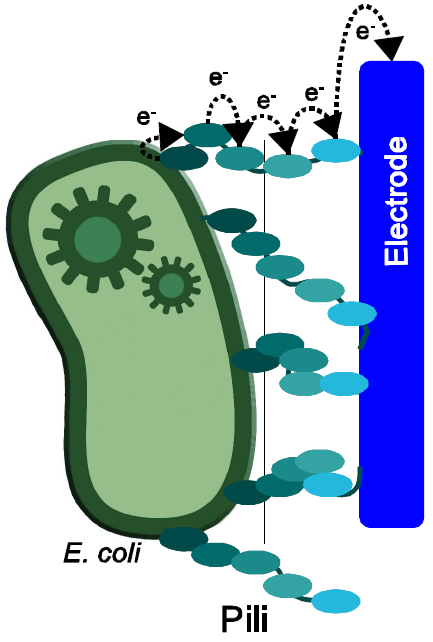
| + | [[Image:Bielefeld-germany-project-overview-nanowires.png|left|thumb|250px|'''Figure 1:''' Principle of electron transfer from bacteria to anode via nanowires.]] | ||
| + | <p align="justify"> | ||
| + | The tetracyclin repressor (TetR)/ operator (TetO) originally is used by E. coli to work against the antibiotic tetracycline but in many cases it is used for regulated expression for industrial processes. When there is no tetracycline available the TetR binds with high affinity the tetracycline operator. When tetracycline is available the TetR switches his conformation and so it comes to a dissolution of the TetR and the TetO. Because of this the polymerase isn’t enhanced anymore and is able to express the genes which lies behind the TetO. In our system the TetR is under the control of a rhamnose promotor (rha-promotor) which only works in the presence of rhamnose. When the bacteria would break out of the MFC there wouldn’t be enough rhamnose in the environment to activate the promotor in a way that enough TetR would be produced to block the polymerase by binding at the TetO. Therefore the polymerase binds to the promotor of TetO and it comes to the expression of RNase Ba and the degradation of the DNA. | ||
| + | </p> | ||
| - | |||
| - | |||
| + | <br><br> | ||
| - | |||
| - | |||
| - | |||
| + | ==Genetic Approach== | ||
| + | |||
| + | [[File:IGEM Bielefeld 2013 biosafety lacI test.png]] | ||
<p align="justify"> | <p align="justify"> | ||
| - | The | + | The lac repressor/operator system uses E.coli to regulate the production of enzymes and because of this E. coli also regulate its metabolic stress. Enzymes only are produced when they are required. LacI is a repressor which is able to inhibit the lac operon in the absence of lactose by binding to the DNA at the lac operator site called lacO. Because of this repressor DNA polymerase is inhibited so it can’t read the sequences behind the operator lacZ (β-galactosidase), lacY (lactose permease) and lacA (thiogalactosidase transacetylase) which are responsible for transporting and metabolism of lactose in E. coli can’t be transcribed. The structure of the lac operon is shown in the image below. |
| - | + | </p> | |
<br> | <br> | ||
| + | [[File:IGEM Bielefeld 2013 Lac operon.png|600px|thumb|center|'''Figure 1:'''Structure of the lactose operon and regulatory units.]] | ||
| - | |||
| - | + | ==Results== | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | ==References== | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | <br><br><br><br> | |
| + | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | < | + | <div id="rightcol" style="width:210px; height:450px; margin-left:752px; margin-top:33px; float:right; position:fixed; overflow-y:scroll; box-shadow:0px 0px 2px 0px grey;" padding:0px 20px;> |
| - | + | __TOC__ | |
| - | + | <div id="spacer" style="height:300px"></div> | |
| - | + | </div> | |
| - | < | + | |
| - | |||
</div> | </div> | ||
Revision as of 23:09, 29 September 2013
Biosafety System Lac of Growth
Overview
The tetracyclin repressor (TetR)/ operator (TetO) originally is used by E. coli to work against the antibiotic tetracycline but in many cases it is used for regulated expression for industrial processes. When there is no tetracycline available the TetR binds with high affinity the tetracycline operator. When tetracycline is available the TetR switches his conformation and so it comes to a dissolution of the TetR and the TetO. Because of this the polymerase isn’t enhanced anymore and is able to express the genes which lies behind the TetO. In our system the TetR is under the control of a rhamnose promotor (rha-promotor) which only works in the presence of rhamnose. When the bacteria would break out of the MFC there wouldn’t be enough rhamnose in the environment to activate the promotor in a way that enough TetR would be produced to block the polymerase by binding at the TetO. Therefore the polymerase binds to the promotor of TetO and it comes to the expression of RNase Ba and the degradation of the DNA.
Genetic Approach
The lac repressor/operator system uses E.coli to regulate the production of enzymes and because of this E. coli also regulate its metabolic stress. Enzymes only are produced when they are required. LacI is a repressor which is able to inhibit the lac operon in the absence of lactose by binding to the DNA at the lac operator site called lacO. Because of this repressor DNA polymerase is inhibited so it can’t read the sequences behind the operator lacZ (β-galactosidase), lacY (lactose permease) and lacA (thiogalactosidase transacetylase) which are responsible for transporting and metabolism of lactose in E. coli can’t be transcribed. The structure of the lac operon is shown in the image below.
Results
References
 "
"