Team:Bielefeld-Germany/Biosafety/Biosafety System L
From 2013.igem.org
Biosafety System Lac of Growth
Overview
comming soon... (will only take a couple of hours)
Genetic Approach
Rhamnose promoter
lacI
Naturally the lac operon regulates the catabolism of the disaccharide lactose (4-O-(β-D-Galactopyranosyl)-D-glucopyranose) in E. coli. The operon contains the lactose promoter (plac) and the genes for the catabolism of the Lactose to Glucose and Galactose. Upstream of the lac operator exists the coding sequence for the repressor lacI under the control of a weak promoter.
Compared to the catabolism of the sugars L-Rhamnose or L-arabinose Lactose, as a disaccharide has a higher energy content and is therefore used more preferable. This is a reason, why the basal transcription of this promoter is even more higher. The leakiness of the lac promoter is caused by the fact that the lacY need to be expressed for an efficient Lactose uptake, while in the arabinose system the uptake is regulated separate.
In our Safety-System the lacI (<bbpart>BBa_C0012</bbpart>) is used for the repression of the lactose promoter (plac).
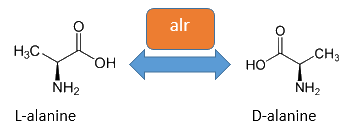
Alanine Racemase
The alanine-racemase alr (EC 5.1.1.1) from the gram-negative enteric bacteria Escherichia coli is a racemase, which catalyses the reversible reaction from L-alanine into the enantiomer D-alanine. For this reaction the cofactor pyridoxal-5'-phosphate (PLP) is typically needed. The constitutive alanine-racemase (alr) is naturally responsible for the accumulation of D-Alanin, which is an essential component of the bacterial cell wall, because it is used for the crosslinkage of the peptidoglykan. The use of D-Alanin instead of a typically L-amino acids prevents the cleavage by peptdidases, but a lack of D-Alanin leeds to a bacteristatic characteristic. So in the absence of D‑Alanine dividing cells will lyse rapidly. So if the expression of the Alanin-Racemase is repressed and there is no D-Alanine-Supplementation in the media, the cells would not increase.
Terminator
Terminator are essential for the end of an operon. In procaryot exists rho-depending and independing terminator. Rho-independing terminators are characterized by an stem-loop, which is caused by special sequence. In general the terminator-region can be divided into four regions. Starting with a GC-rich region, which performs the stem and followed by the loop-region. The third region is made up from the opposite part of the stem, so that this region concerns also GC-rich portion. After that the terminator ends by an poly uracil region, which destabilizes the binding of the RNA-polymerase. The stem-loop of the terminator causes a distinction of the DNA and the translated RNA, so that the binding of the RNA-polymerase is canceld and the transcription ends after the stem-loop (Carafa et al., 1990).
For our Safety-System the terminator is necessary to avoid that the expression of the genes under the control of the Rhamnose promoter pRHA, like the Repressor araC and the Alanine-Racemase (alr), are transcripted but not the genes of the Arabinose promoter pBAD, which contains the toxic Barnase and would lead to cell death.
Lactose promoter (plac)
Naturally the lac operon regulates the catabolism of the disaccharide lactose (4-O-(β-D-Galactopyranosyl)-D-glucopyranose) in E. coli. The operon consists of a CAP-binding site, the lac promoter, the lac operator and the genes lacZ, lacY and lacA downstream of the promoter. The transcription of the lactose promoter is regulated by the lacI gene, which is found upstream of the operon under the control of a weak promoter. In the absence of lactose the transcription of the genes behind the lactose promoter is blocked caused by the binding of the lacI pressor. While in the presence of Lactose the repressor is released from the operator and the genes can be transcripted. Typically the transcription is enhanced by a high intracellular level of cAMP.
Barnase
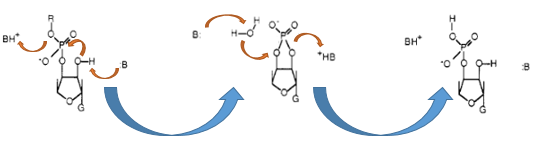
The Barnase (EC 3.1.27) is an 12 kDa extracellular microbial ribonuclease, which is naturally found in the gram-positive soilbacteria Bacillus amyloliquefaciens and consist a single chain of 110 amino acids. The Barnase (RNase Ba) catalyses the cleavage of single stranded RNA, where the hydrolysis of the dinucleotides has the highest affinity to the structure GpN. In the first step of the RNA-degradation a cyclic intermediate is formed by transesterification and afterwards this intermediate is hydrolysed yielding in a 3'-nucleotide (see graphic below).
In Bacillus amyloliquefaciens the activity ot the Barnase (RNase Ba) is inhibited intracelluar by the Inhibitor called barstar. Barstar consists only about 89 amnio acids and binds with a high affinity to the toxic Barnase. This prevents the cleavage of the intracellular RNA in the host organismen. Therefore the Barnase acts naturally only outside the cell and is translocated under natural conditions. For the Biosafety-System we tried to modified this aspect by cloning only the sequence responsbile for the cleavage of the RNA, but not the part of the native Barnase, which is essential for the extracellular transport.
As shown in the graphic below, the transcription of the DNA, which encodes the Barnase produces a 474 nt RNA. The translation of the RNA of this ribonuclease starts about 25 nucleotids downstream from the transcription start and can be divided into two parts. The first part (colored in orange) is translated into a signal peptide at the N-Terminus of the Barnase. This part is resoponsible for the extracellular translocation of the RNase Ba, while the peptid sequence for the active Barnase starts 142 nucleotides downstream from the transcription start (colored in red). For the Biosafety-System we used only the coding sequence of the Barnase itself to prevent the extracellular translocation of the toxic gene product. This leds to a rapid cell death if the expression of the Barnase isn't repressed by the repressor of the Biosafety-System.
Results
References
Agnes Ullmann (2001): Escherichia coli Lactose Operon. In: Encyclopedia of Life Sciences
Stumpp et al.: Ein neues, L-Rhamnose-induzierbares Expressionssystem für Escherichia coli, In: Biospektrum 6. Jahrgang S. 33
Carsten Voss, Dennis Lindau, and Erwin Flaschel, Production of Recombinant RNase Ba and Its Application in Downstream Processing of Plasmid DNA for Pharmaceutical Use, Biotechnology Progress, 22, 2006 p. 737-44.
Danuta E. Mossakowska, Kerstin Nyberg, and Alan R. Fersht, Kinetic Characterization of the Recombinant Ribonuclease from Bacillus amyloliquefaciens (Barnase) and Investigation of Key Residues in Catalysis by Site-Directed Mutagenesis, Biochemistry, 28, 1989, p. 3843 – 3850.
C. J. Paddon, N. Vasantha, and R. W. Hartley, Translation and Processing of Bacillus amyloliquefaciens Extracellular Rnase, Journal of Bacteriology, 171, 1989, p. 1185 – 1187.
- Autoren (Jahr) Titel [Link|Paper Ausgabe: Seiten].
 "
"