Team:Bielefeld-Germany/Biosafety/Biosafety System
From 2013.igem.org
| Line 87: | Line 87: | ||
[[File:IGEM Bielefeld 2013 Biosafety allg. systemaufbau.png|600px|thumb|center|FigureX:...]] | [[File:IGEM Bielefeld 2013 Biosafety allg. systemaufbau.png|600px|thumb|center|FigureX:...]] | ||
| - | + | <br> | |
<p align="justify"> | <p align="justify"> | ||
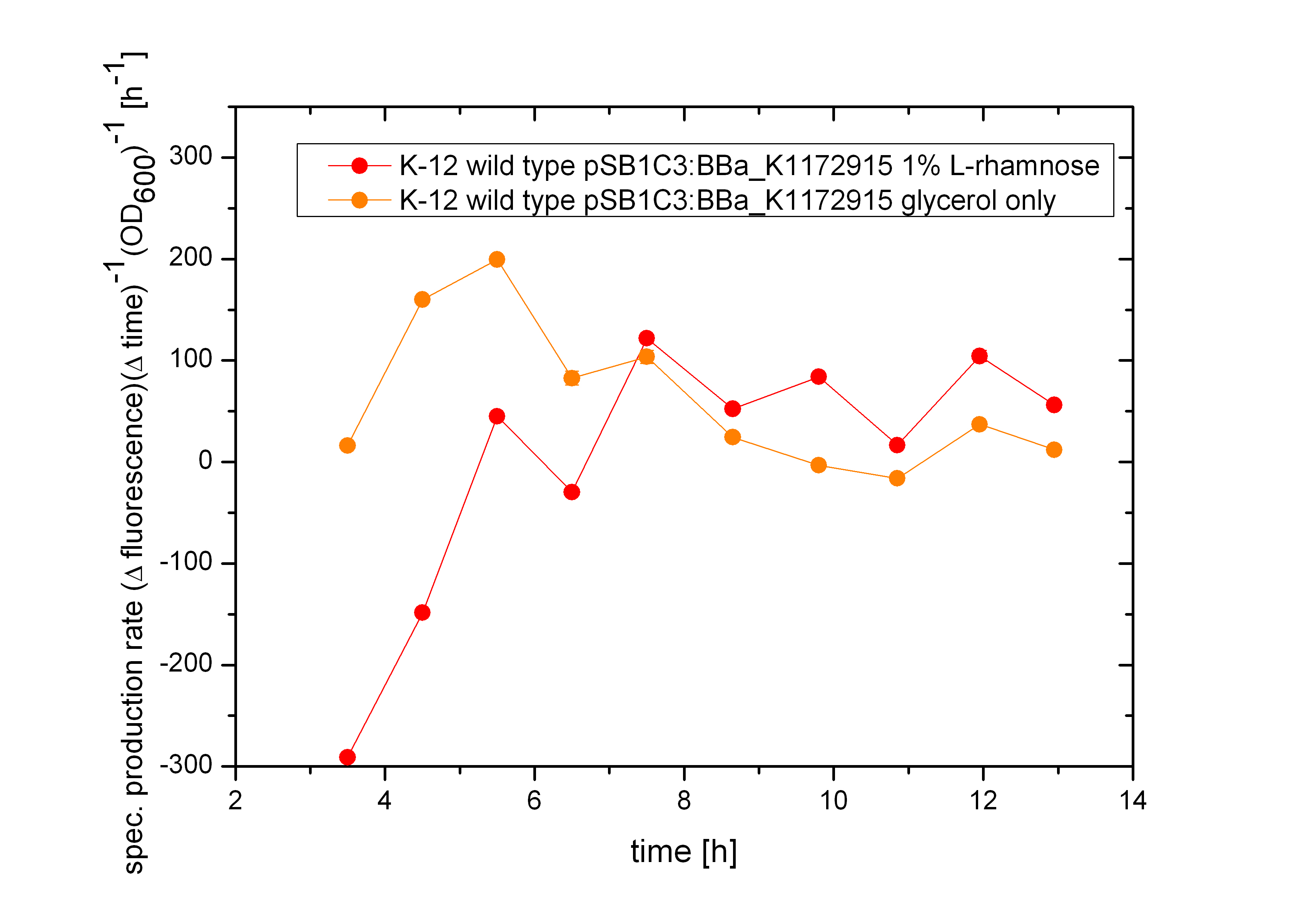
| + | As shown in figure 2 above, the Biosafety-Strain survives only under the defined conditions by supplementation of L-Rhamnose to the media. L-Rhamnose induces the expression of the Alanin- Racemase, so that the bacteria can grow and the expression of the repressor, which inhibits the expression of the toxic Barnase. To establish a functional Biosafety-System we first compared three different System (araCtive, TetOR alive and Lac of Growth) by variations of the repressor and the corresponding promoter to measure the basal transcription and repression of the second part. This is necessary, for the first fine tuning of the Biosafety-System, as it was not clear how high the expression level of the Barnase could be without causing cell death. With GFP it was therefore possible to become a first impression of how low the transcription of the Barnase would be in the repressed situation.<br> | ||
| + | In contrast, when there is no L-Rhamnose present in the media or the concentration of L-Rhamnose decreases because of the natural rhmanose catabolism of the Biosafety-Strain K-12 ∆alr ∆dadX ∆araC, the expression of the first part with the Alanine-Racemase (''alr'') and the repressor decreases (see figure 3 below). This leads to an activation of the second promoter. Also for this situation the testing with GFP was necessary before the usage of the toxic Barnase. In this case for the reason to become an impression how high the expression of the Barnase would be.</p><br> | ||
| + | |||
| + | [[File:IGEM Bielefeld 2013 Biosafety allg. systemaufbauohnerhamnose.png|600px|thumb|center|FigureX:...]] | ||
<br> | <br> | ||
| - | + | <p align="justify"> | |
[[File:IGEM Bielefeld 2013 Biosafety alrkillwennbarnasemutiert.png|600px|thumb|center|FigureX:...]] | [[File:IGEM Bielefeld 2013 Biosafety alrkillwennbarnasemutiert.png|600px|thumb|center|FigureX:...]] | ||
| - | + | ||
==Results== | ==Results== | ||
Revision as of 02:32, 5 October 2013
Biosafety System
Overview
Biosafety is an important aspect of Synthetic Biology, because it deals with the interaction of the environment and mankind. Several studies deal with the interaction of genetically modified bacteria and natural wild types. Mostly with the result that genetically modified bacteria does not influence the environment, but there is always a risk remaining. Because the genetically modified bacteria are adapted to the excellent conditions of the laboratory, the natural bacteria will outlast this modified strains in nature. So the genetically modified bacteria will not survive because of these evolutionary disadvantage. But there is no guarantee that there is really no interaction and that their release does not effect the equilibrium of the environment. To avoid these problems we also thought of a cell free fuel cell based on enzymes, but this is even more complex and would not be as efficient because of the limited activity of the enzymes. So the questions was, how will an approach as our microbial fuel cell, with genetic modified bacteria for a higher production of energy find application outside the laboratory, without effecting the nature?
The answers of this question can be found in our Safety-System. This now approach opens the possibility to control the cell division of living bacteria in a defined environment like the MFC. The Biosafety-System ensures, that the genetically modified bacteria can only survive in the defined zone, while they die, when they leave this area by accident or damage of the MFC chassi.
Briefly the basic of our Biosafety-System takes advantage of two common Biosafety-ideas, an auxotrophic strain and a toxic gene product. Our approach combines both in only one device. The so constructed Biosafety-System takes the best of this two approaches by establishing an new useful auxotrophy and a new toxic gene product in the partsregistry and is additional characterized as the first registered double kill-switch system. By using two different kill-switches, each individual switch can be a bck-up and our Biosafety-System provides additional a higher plasmid stability and a higher resistance towards undesirable mutations in the Safety-System. In one sentence: Our Biosafety-System is safe!
Theory
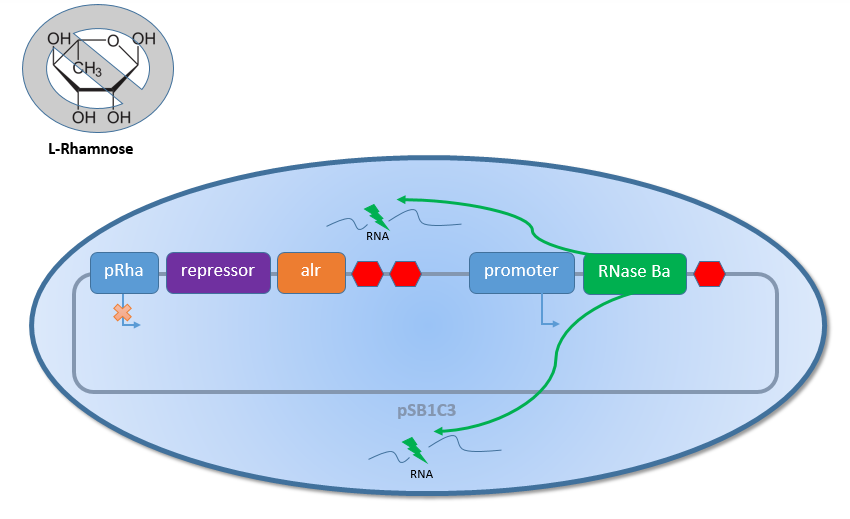
The auxotrophic Safety-Strain <link> E. coli K-12 ∆alr ∆dadX ∆araC is the basic of our three Safety-Systems Lac of growth <link>, TetOR alive <link> and araCtive <link>. In this new constructed Safety-Strain the constitutive Alanine-Racemase (alr) and the catabolic Alanine- Racemase (dadX) are deleted. As the Alanine-Racemase catalyses the reversible isomerization from L-alanine into the enantiomer D-alanine the Safety-strain is no longer able to synthesis the essential amino acid D-alanine. D-alanine is an essential molecule for the Gram-negative bacteria E. coli, because it is responsible for the cross-linkage of the peptidoglycanlayer, so that a lack of D-alanine inhibits cell division and leads to cell lysis in growing bacteria. This effect is comparable to bacteristatic characteristic known from beta-lactam-antbiotics.
By complementation of the Alanine-Racemase (alr) via plasmid, the supplementation of D-alanine is not any more necessary for the Biosafety-Strain <link>. But as the complementation is essential for bacterial cell division it can be used as an antibiotic-free selection System.
When expressing toxic gene products like the Rnase Ba (Barnase) or Colicin E2 the maintenance of the plasmid stability is a very important parameter. And in a Biosafety-System it is even more important then in industrial cultivation, because the loose of the plasmid containing the Biosafety- System causes failing of the whole Biosafety-System. This situation is avoided in the Biosafety- System by the complementation of the Alanine-Racemase in the Biosafety-Strain K-12 ∆alr ∆dadX ∆araC. If these mutants will loose the plasmid, caused by the stress of the toxic gene product, they will not be able to grow because the bacteria can not synthesize D-alanine for the peptidoglycan- layer of the cell wall.
In general the Biosafety-System can be divided into two parts, which are regulated by different inducible promoters. The first part contains the essential Alanine-Racemase (alr) and the corresponding repressor for the promoter of the second part. Both genes a regulated by the rhamnose inducible promoter pRhaBAD (<bbpart>BBa_K914003</bbpart>). The second part of the Biosafety-System contains another promoter which depends on the Safety-System. The Biosafety-System araCtive as example uses the arabinose promoter pBAD. This promoter controls
in the final version the expression of the toxic RNAse Ba (<bbpart>BBa_K1172904</bbpart>), but for the testing and comparison of the different Biosafety-Systems GFP (<bbpart>BBa_E0040</bbpart>) was used in all of the Systems instead. So a selection of the best designed Biosafety-System is possible without directly natural selection by the Biosafety-System himself.
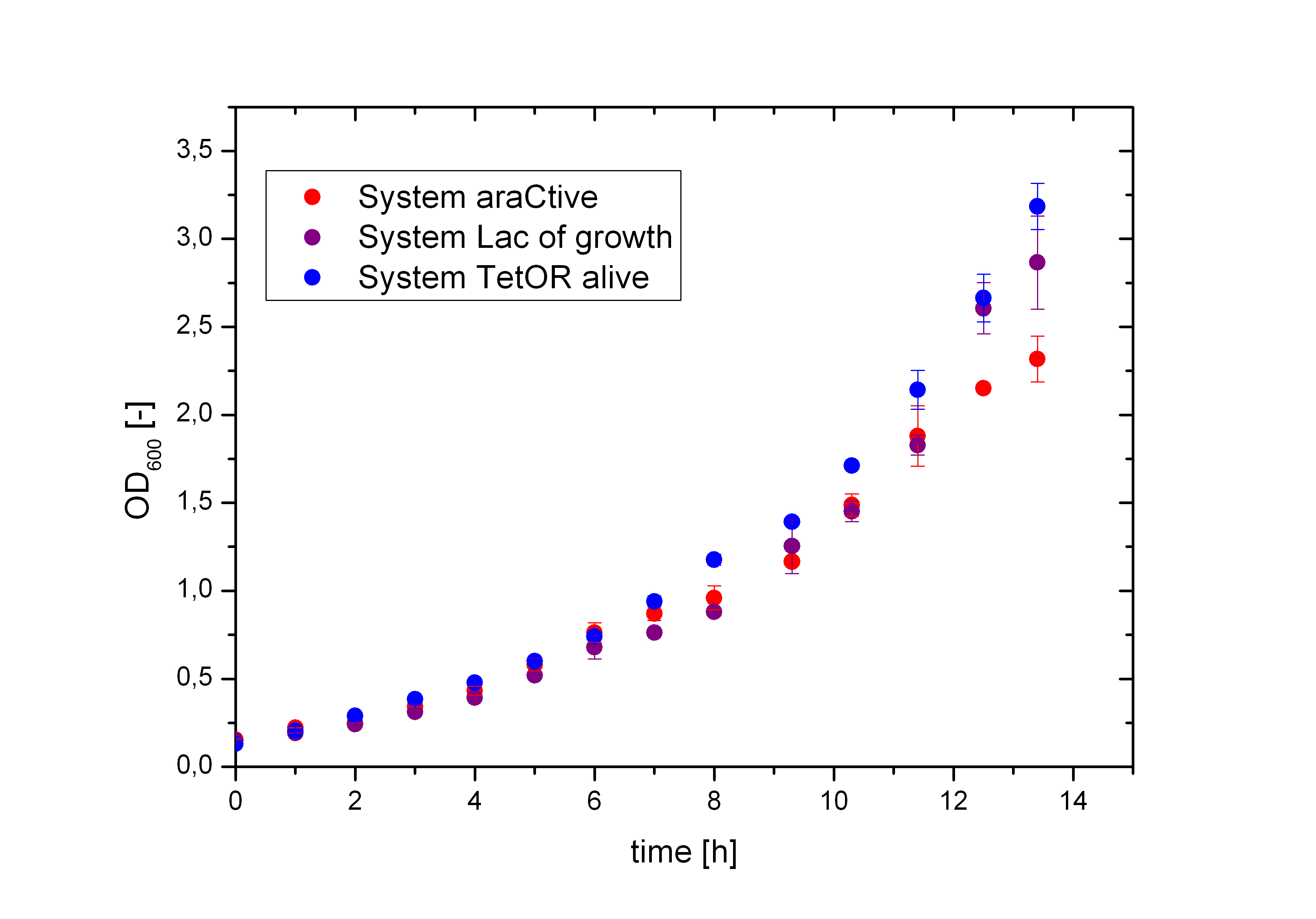
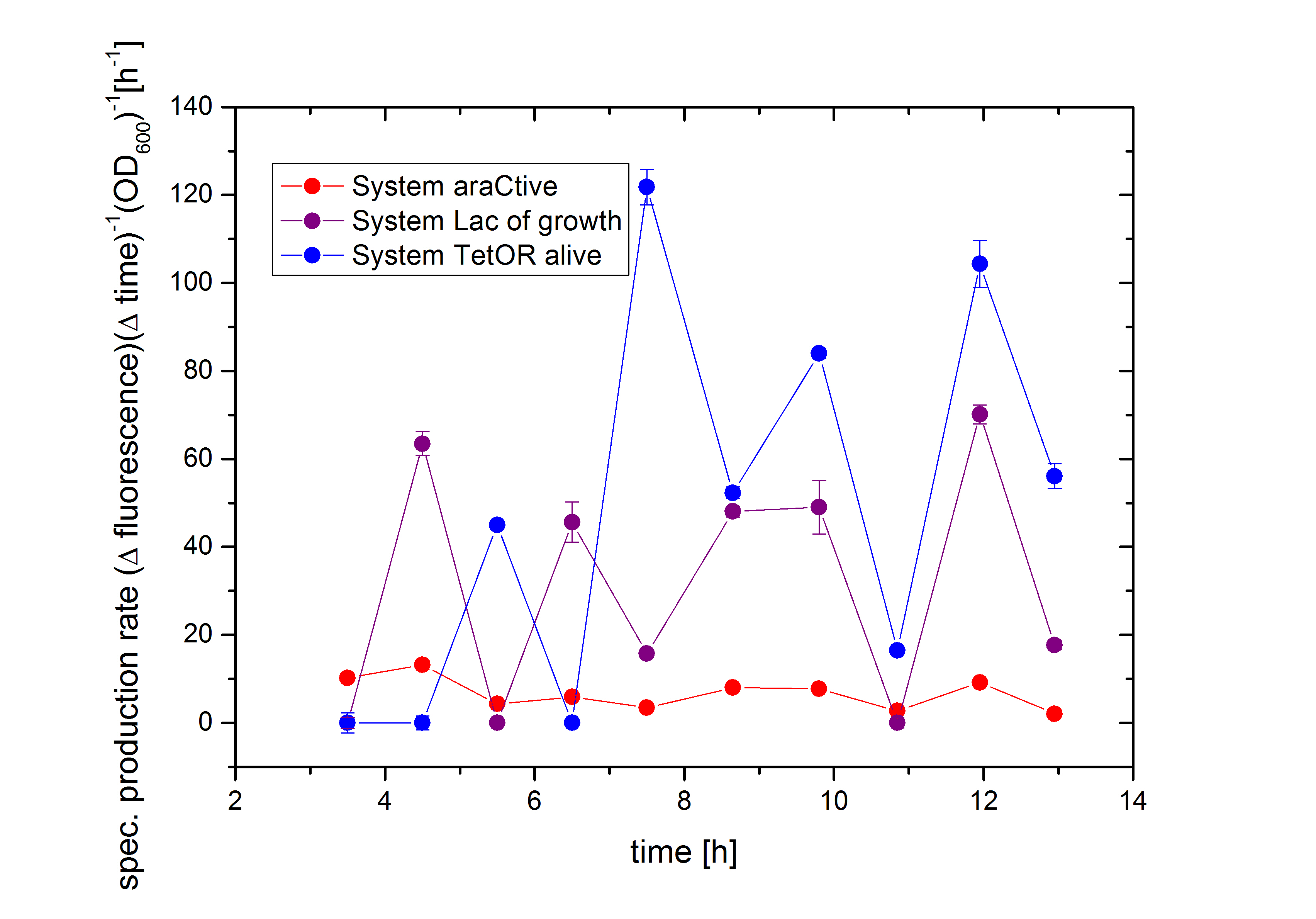
As shown in figure 2 above, the Biosafety-Strain survives only under the defined conditions by supplementation of L-Rhamnose to the media. L-Rhamnose induces the expression of the Alanin- Racemase, so that the bacteria can grow and the expression of the repressor, which inhibits the expression of the toxic Barnase. To establish a functional Biosafety-System we first compared three different System (araCtive, TetOR alive and Lac of Growth) by variations of the repressor and the corresponding promoter to measure the basal transcription and repression of the second part. This is necessary, for the first fine tuning of the Biosafety-System, as it was not clear how high the expression level of the Barnase could be without causing cell death. With GFP it was therefore possible to become a first impression of how low the transcription of the Barnase would be in the repressed situation.
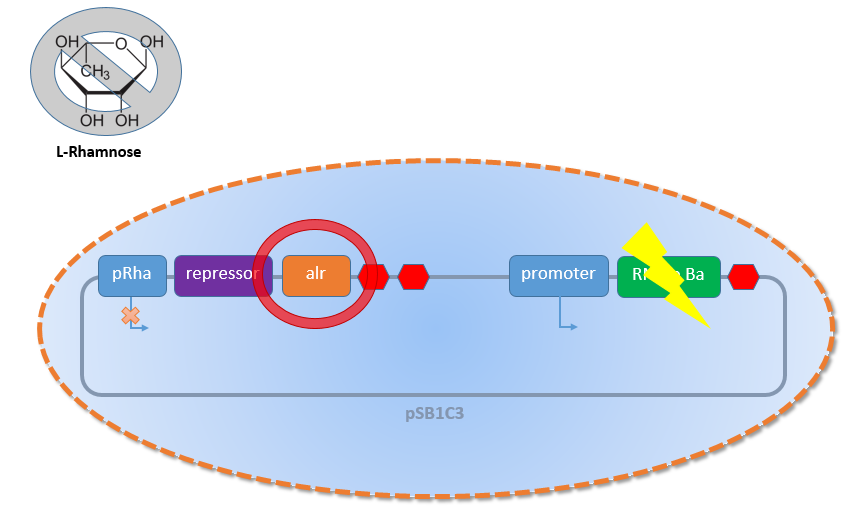
In contrast, when there is no L-Rhamnose present in the media or the concentration of L-Rhamnose decreases because of the natural rhmanose catabolism of the Biosafety-Strain K-12 ∆alr ∆dadX ∆araC, the expression of the first part with the Alanine-Racemase (alr) and the repressor decreases (see figure 3 below). This leads to an activation of the second promoter. Also for this situation the testing with GFP was necessary before the usage of the toxic Barnase. In this case for the reason to become an impression how high the expression of the Barnase would be.
Results
References
- Autoren (Jahr) Titel [Link|Paper Ausgabe: Seiten].
</div>
 "
"