Team:Bielefeld-Germany/Biosafety/Biosafety System L
From 2013.igem.org
Biosafety System Lac of Growth
Overview
comming soon... (will only take a couple of hours)
Genetic Approach
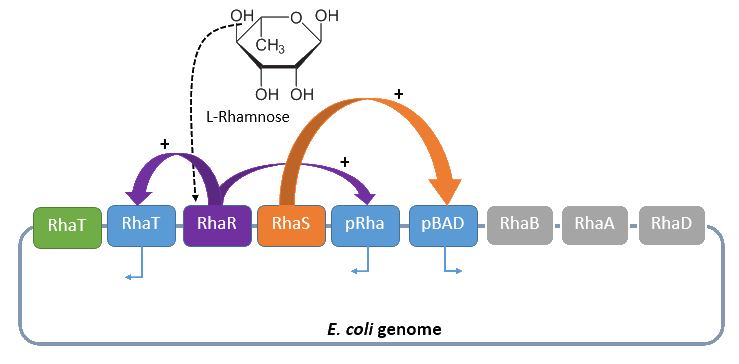
Rhamnose promoter
The promoter rhaBAD regulates naturally the catabolism of the hexose L-rhamnose. The advantage of the operon is its positively regulation. All in all the regulon consists of the promoter pRhaT, who regulates the expression of the protein RhaT for the uptake of L-Rhamnose, the operons rhaSR and rhaBAD.
The operon rhaSR regulates the genes RhaS and RhaR, whose translated proteins are responsible for the positive activation of the L-Rhamnose catabolism, while the operon rhaBAD regulates the genes for the direct catabolism of L-Rhamnose.
When L-rhamnose is present, it acts as an inducer by binding to the regulatory protein RhaR. RhaR regulates his own expression and the expression of the regulatory gene RhaS by inhibiting or activating, in the presence of L-Rhamnose, the operon RhaSR. Normally the expression level is modest, but it can be enhanced by a higher level of intracellular cAMP, which increases in the absence of glucose. So in the presence of L-Rhamnose and a high concentration of intracellular cAMP the activor protein RhaR is expressed on higher level, resulting in an activation of the promoter pRhaT for an efficient L-rhamnose uptake and an activation of the operon rhaBAD. The L-Rhamnose is than broken down into dihydroxyacetone phosphate and lactate aldehyde by the enzymes of the operon rhaBAD (Wickstrum et al., 2005).
Dihydroxyacetone phosphate can be metabolized in the glycolysis pathway, while the lactate aldehyde is oxized to lactate under aerobic conditions and reduced to L-1,2,-propandiol under anaerobic conditions.
This degradation of L-Rhamnose can be separated in three steps. In the first step the L-Rhamnose is turned into L-Rhamnulose by an isomerase (gene RhaA). The catabolism continues by the a kinase (gene RhaB), who phosphorylates the L-Rhamnulose to L-Rhamnulose-1-phosphate. This is finally hydrolyzed by Aldolase (gene RhaD) to dihydroxyacetone phosphate and lactate aldehyde (Baldoma et al., 1988).
Four our Safety-System the rhamnose promoter rhaBAD is used to control the expression of the repressor araC and the essential Alanine-Racemase, because this promoter has even a lower basal transcription then the arabinose promoter pBAD. This is needed to tightly repress the expression of the Alanine-Racemase (alr) and take so advantage of the double-kill switch. So although the rhamnose promoter rhaBAD is characterized by a very low basal transcription it can not be used for the control of the toxic Barnase, because the regulation is mainly organized by an activation and not an active repression, but this is optimal for the first containing the Alanine-Racemase. So in conclusion this promoter is the best choice for the first part of the Biosafety-System, as it is tightly repressed in the absence of L-Rhamnose, but activated in its presence.
lacI
Naturally the lac operon regulates the catabolism of the disaccharide lactose (4-O-(β-D-Galactopyranosyl)-D-glucopyranose) in E. coli. The operon contains the lactose promoter (plac) and the genes for the catabolism of the Lactose to Glucose and Galactose. Upstream of the lac operator exists the coding sequence for the repressor lacI under the control of a weak promoter (Jacob et al., 1961).
Compared to the catabolism of the sugars L-Rhamnose or L-arabinose Lactose, as a disaccharide has a higher energy content and is therefore used more preferable. This is a reason, why the basal transcription of this promoter is even more higher. The leakiness of the lac promoter is caused by the fact that the lacY need to be expressed for an efficient Lactose uptake, while in the arabinose system the uptake is regulated separate (Görke et al., 2008).
In our Safety-System the lacI (<bbpart>BBa_C0012</bbpart>) is used for the repression of the lactose promoter (plac).
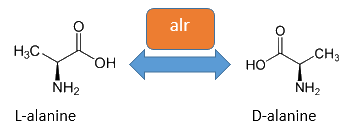
Alanine Racemase
The alanine-racemase alr (EC 5.1.1.1) from the gram-negative enteric bacteria Escherichia coli is a racemase, which catalyses the reversible reaction from L-alanine into the enantiomer D-alanine. For this reaction the cofactor pyridoxal-5'-phosphate (PLP) is typically needed. The constitutive alanine-racemase (alr) is naturally responsible for the accumulation of D-Alanin, which is an essential component of the bacterial cell wall, because it is used for the crosslinkage of the peptidoglykan (Walsh, 1989).
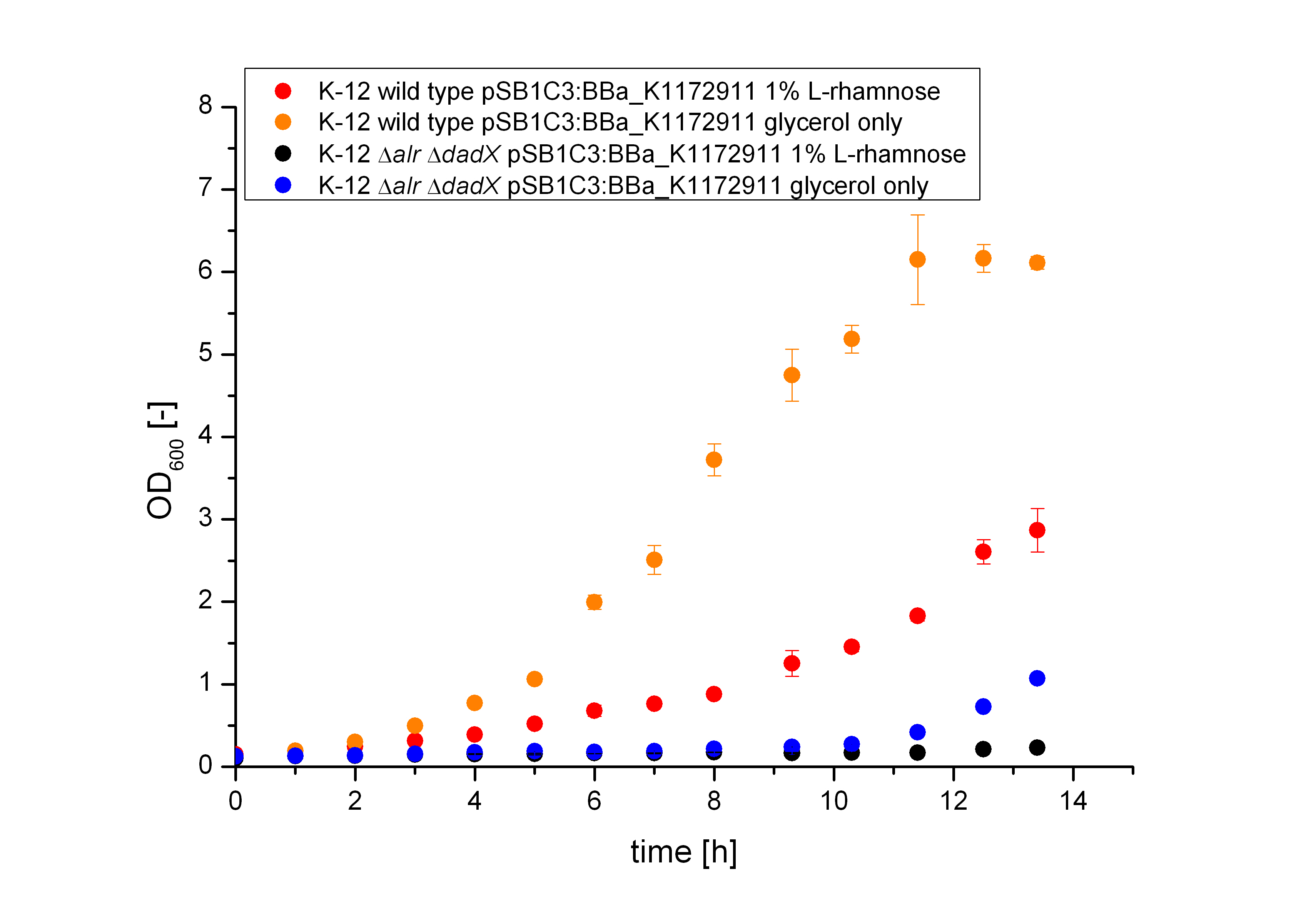
The use of D-Alanine instead of a typically L-amino acids prevents the cleavage by peptdidases, but a lack of D-Alanine leeds to a bacteriostatic characteristic. So in the absence of D‑Alanine dividing cells will lyse rapidly. This approach is used by our Biosafety-Strain, a D-alanine auxotrophic mutant (K-12 ∆alr ∆dadX). The Safety-Strain grows only with a plasmid containing the Alanine-Racemase (<bbpart>BBa_K1172901</bbpart>) for the complementation of the D-alanine auxotrophic. Because the Alanine-Racemase is therefore essential for bacterial cell division, this approach guarantees a high plasmid stability, which is extremely important when the plasmid contains a toxic gene like the Barnase. In addition this construction provides the possibility of a double kill-switch system. Because if the expression of the Alanine-Racemase is repressed and there is no D-Alanine-Supplementation in the media, the cells would not increase.
Terminator
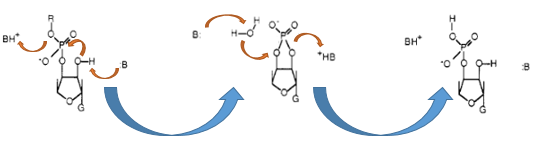
Terminator are essential for the end of an operon. In procaryot exists rho-depending and independing terminator. Rho-independing terminators are characterized by an stem-loop, which is caused by special sequence. In general the terminator-region can be divided into four regions. Starting with a GC-rich region, which performs the stem and followed by the loop-region. The third region is made up from the opposite part of the stem, so that this region concerns also GC-rich portion. After that the terminator ends by an poly uracil region, which destabilizes the binding of the RNA-polymerase. The stem-loop of the terminator causes a distinction of the DNA and the translated RNA, so that the binding of the RNA-polymerase is canceld and the transcription ends after the stem-loop (Carafa et al., 1990).
For our Safety-System the terminator is necessary to avoid that the expression of the genes under the control of the Rhamnose promoter pRHA, like the Repressor araC and the Alanine-Racemase (alr), are transcripted but not the genes of the Arabinose promoter pBAD, which contains the toxic Barnase and would lead to cell death.
Lactose promoter (plac)
Naturally the lac operon regulates the catabolism of the disaccharide lactose (4-O-(β-D-Galactopyranosyl)-D-glucopyranose) in E. coli. The operon consists of a CAP-binding site, the lac promoter, the lac operator and the genes lacZ, lacY and lacA downstream of the promoter. The transcription of the lactose promoter is regulated by the lacI gene, which is found upstream of the operon under the control of a weak promoter. In the absence of lactose the transcription of the genes behind the lactose promoter is blocked caused by the binding of the lacI pressor. While in the presence of Lactose the repressor is released from the operator and the genes can be transcripted. Typically the transcription is enhanced by a high intracellular level of cAMP (Busby, 1999).

The lactose promoter thereby regulates the transcription of the genes lacZ, lacY and lacA. The lacZ gene encodes for the ß-Galactosidase a enzyme, who breaks down the lactose to glucose and galactose. The ß-Galactosidase catalyses additional the degradation from Lactose to Allolactose. By binding on the lacI repressor, it changes his conformation an is not any more able to bind on the operator sequnece and to block the transcription. As only one enzyme is necessary to gain a substrate of the glycolysis is becomes clear, why the degradation of Lactose is more preferable compared to L-arabinose or L-rhamnose.
To realize a preference of lactose, the transcription of the lactose promoter is not repressed as that strong. This is caused by the fact that the lacY gene, coding for the integral membrane protein lactose permease, is necessary for the lactose uptake and has to be transcripted on a low level.
The last gene of the lac operon, lacA, encodes for a Transacetylase, who acetylizes glycosides that can not be metabolized. The acetylated glycosides are transported outside the cell to avoid the accumulation of lactose.
In our Biosafety-System the lac-promoter is used for the regulation of GFP or the Barnase. As the lac promoter shows a high basal transcription, its might not ideal for the regulation of a toxic gene product, but the the Biosafety-System Lac of growth is ideal for comparison with the other Systems to measure the level of basal transcription under repressed and unrepressed conditions. Besides we improved the leakiness of the lactose promoter by adding a second lacI-binding site 12 nt downstream of the excisting bining site. As this distance corresponds to about one whorl of the double helix, this should allow an additional lacI repressor to bind on the other site of the DNA and tighten the repression of the lactose promoter. Unfortunately the improvement of the so called double lac promoter could not be quantified, because lac of time (Lewis, 2005).
Barnase
The Barnase (EC 3.1.27) is an 12 kDa extracellular microbial ribonuclease, which is naturally found in the gram-positive soil bacteria Bacillus amyloliquefaciens and consist a single chain of 110 amino acids. The Barnase (RNase Ba) catalyses the cleavage of single stranded RNA, where the hydrolysis of the dinucleotides has the highest affinity to the structure GpN. In the first step of the RNA-degradation a cyclic intermediate is formed by transesterification and afterwards this intermediate is hydrolysed yielding in a 3'-nucleotide (Mossakowska et al., 1989).
In Bacillus amyloliquefaciens the activity ot the Barnase (RNase Ba) is inhibited intracelluar by the Inhibitor called barstar. Barstar consists only about 89 amino acids and binds with a high affinity to the toxic Barnase. This prevents the cleavage of the intracellular RNA in the host organism (Paddon et al., 1989). Therefore the Barnase acts naturally only outside the cell and is translocated under natural conditions. For the Biosafety-System we tried to modified this aspect by cloning only the sequence responsible for the cleavage of the RNA, but not the part of the native Barnase, which is essential for the extracellular transport.
As shown in the graphic below, the transcription of the DNA, which encodes the Barnase produces a 474 nt RNA. The translation of the RNA of this ribonuclease starts about 25 nucleotides downstream from the transcription start and can be divided into two parts. The first part (colored in orange) is translated into a signal peptide at the N-Terminus of the Barnase. This part is responsible for the extracellular translocation of the RNase Ba, while the peptide sequence for the active Barnase starts 142 nucleotides downstream from the transcription start (colored in red).
For the Biosafety-System we used only the coding sequence (<bbpart>BBa_K1172904</bbpart>) of the Barnase itself to prevent the extracellular translocation of the toxic gene product. This leads to a rapid cell death if the expression of the Barnase isn't repressed by the repressor of the Biosafety-System.
Results
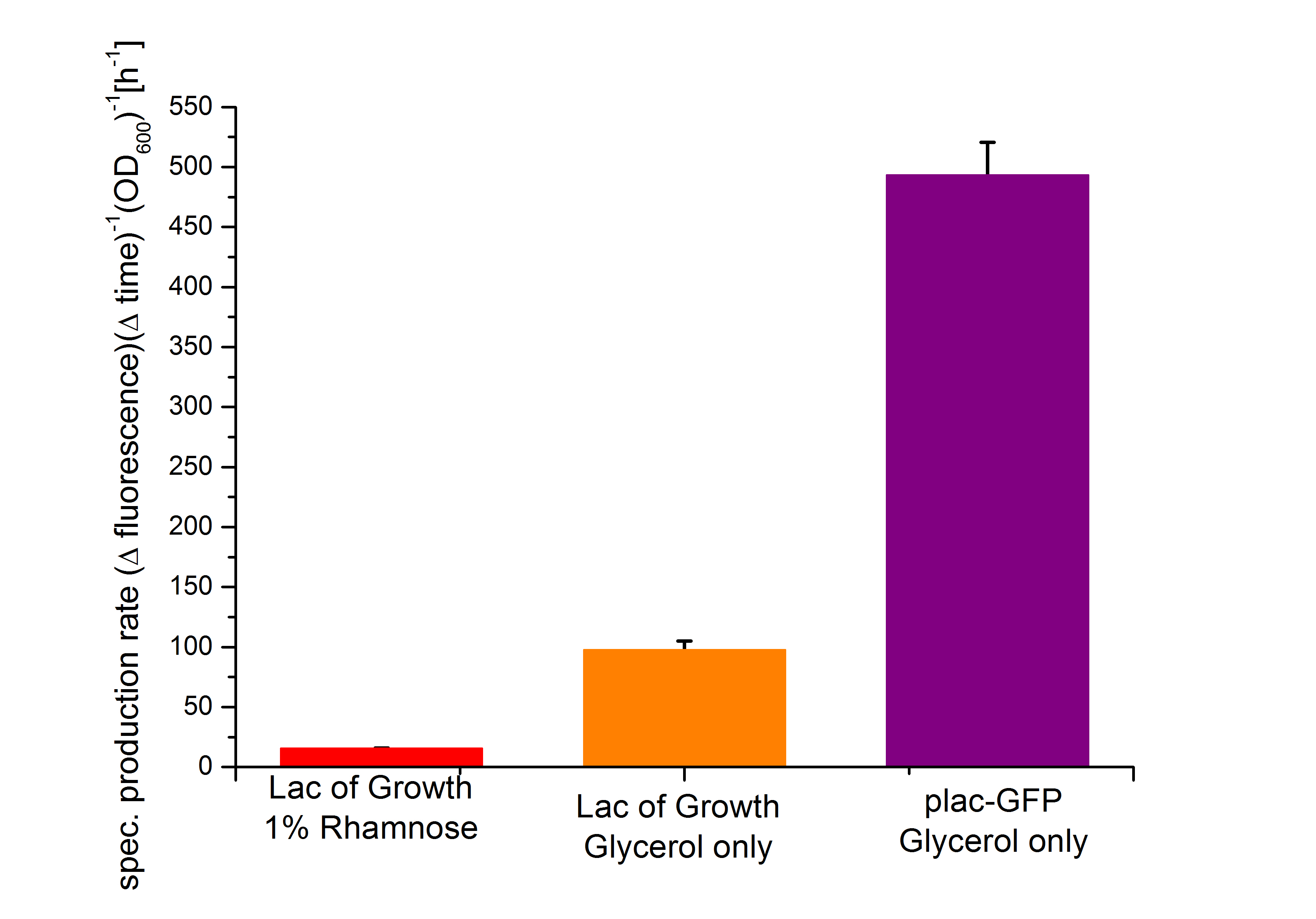
Characterization of the lactose promoter plac
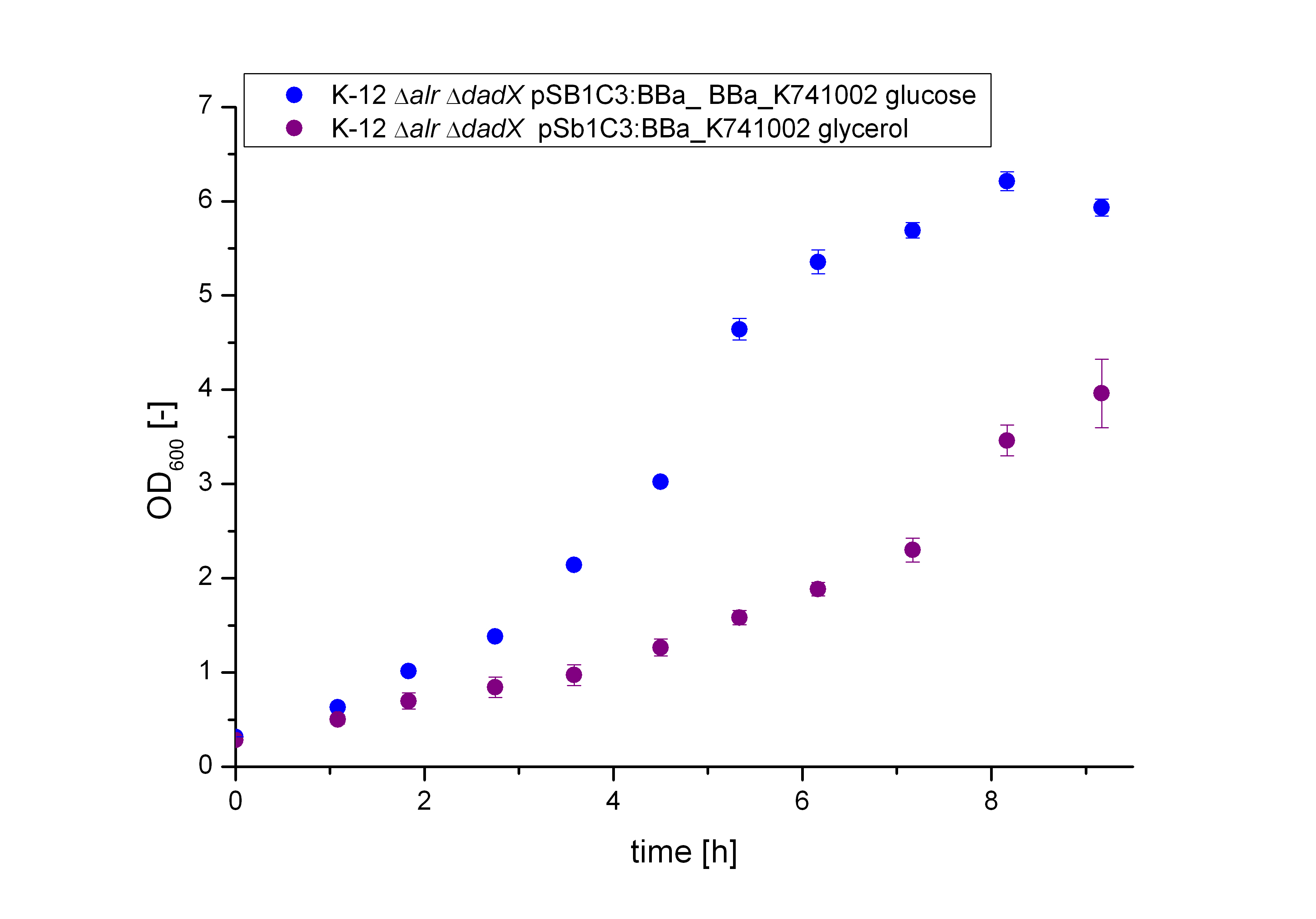
Characterization of the Biosafety-System Lac of growth
Conclusion of the Results
References
- Baldoma L and Aguilar J (1988) Metabolism of L-Fucose and L-Rhamnose in Escherichia coli: Aerobic-Anaerobic Regulation of L-Lactaldehyde Dissimilation [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC210658/pdf/jbacter00179-0434.pdf|Journal of Bacteriology 170: 416 - 421.].
- Busby S, Ebright RH (1999) Transcription activation by catabolite activator protein (CAP).[http://ac.els-cdn.com/S0022283699931613/1-s2.0-S0022283699931613-main.pdf?_tid=a8f22b74-2cf4-11e3-9457-00000aab0f6c&acdnat=1380891687_f17fd24e5a3e15da96493226fdcaaa10|Journal of molecular biology 239: 199 - 213.].
- Carafa, Yves d'Aubenton Brody, Edward and Claude (1990) Thermest Prediction of Rho-independent Escherichia coli Transcription Terminators - A Statistical Analysis of their RNA Stem-Loop Structures [http://ac.els-cdn.com/S0022283699800059/1-s2.0-S0022283699800059-main.pdf?_tid=ede07e2a-2a92-11e3-b889-00000aab0f6c&acdnat=1380629809_2d1a59e395fc69c8608ab8b5aea842f7|Journal of molecular biology 3: 835 - 858].
- Görke, Boris and Stülke, Jörg (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients [http://www.nature.com/nrmicro/journal/v6/n8/full/nrmicro1932.html|Nature Reviews Microbiology 6: 613 - 624].
- Jacob, F Monod J (1961) Genetic regulatory mechanisms in the synthesis of proteins. [http://www.ncbi.nlm.nih.gov/pubmed/13718526|Journal of molecular biology 216: 318 - 356].
- Lewis, Mitchell (2005) The lac repressor [http://www.sciencedirect.com/science/article/pii/S1631069105000685|Comptes rendues biologies 328: 521 – 548].
- Mossakowska, Danuta E. Nyberg, Kerstin and Fersht, Alan R. (1989) Kinetic Characterization of the Recombinant Ribonuclease from Bacillus amyloliquefaciens (Barnase) and Investigation of Key Residues in Catalysis by Site-Directed Mutagenesis [http://pubs.acs.org/doi/pdf/10.1021/bi00435a033|Biochemistry 28: 3843 - 3850.].
- Paddon, C. J. Vasantha, N. and Hartley, R. W. (1989) Translation and Processing of Bacillus amyloliquefaciens Extracellular Rnase [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC209718/pdf/jbacter00168-0575.pdf|Journal of Bacteriology 171: 1185 - 1187.].
- Voss, Carsten Lindau, Dennis and Flaschel, Erwin (2006) Production of Recombinant RNase Ba and Its Application in Downstream Processing of Plasmid DNA for Pharmaceutical Use [http://onlinelibrary.wiley.com/doi/10.1021/bp050417e/pdf|Biotechnology Progress 22: 737 - 744.].
- Walsh, Christopher (1989) Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. [http://www.jbc.org/content/264/5/2393.long|Journal of biological chemistry 264: 2393 - 2396.]
- Wickstrum, J.R., Santangelo, T.J., and Egan, S.M. (2005) Cyclic AMP receptor protein and RhaR synergistically activate transcription from the L-rhamnose-responsive rhaSR promoter in Escherichia coli. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251584/?report=reader|Journal of Bacteriology 187: 6708 – 6719.].
Contents |
 "
"