Team:Bielefeld-Germany/Biosafety/Safety-Form
From 2013.igem.org
(Difference between revisions)
| Line 3: | Line 3: | ||
__NOTOC__ | __NOTOC__ | ||
| + | |||
<div id=globalwrapper style="padding-left:20px; padding-right:20px"> | <div id=globalwrapper style="padding-left:20px; padding-right:20px"> | ||
Latest revision as of 00:46, 5 October 2013
Biosafety Form
Basic Safety Questions for iGEM 2013
- 1. Please describe the chassis organism(s) you will be using for this project. If you will be using more than one chassis organism, provide information on each of them:
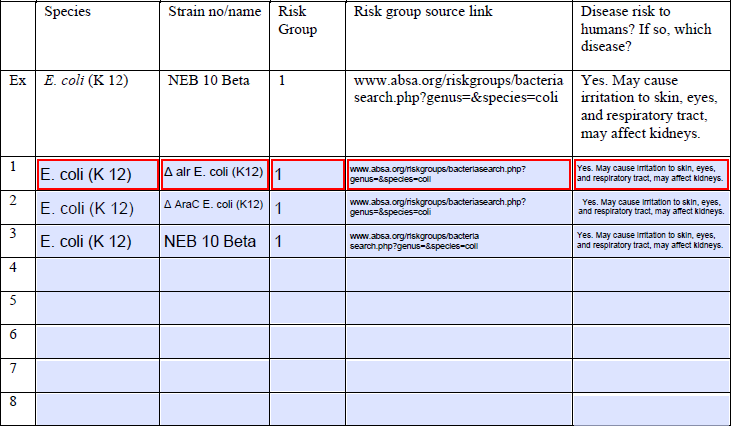
- 2. Highest Risk Group Listed:
- The highest risk group is one.
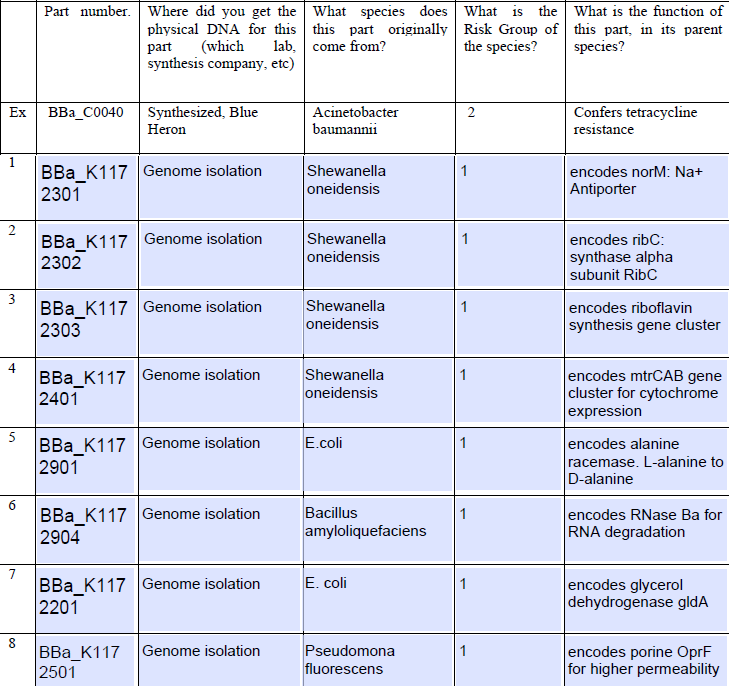
- 3. List and describe all new or modified coding regions you will be using in your project.
- 4. Do the biological materials used in your lab work pose any of the following risks? Please describe.
- a. Risks to the safety and health of team members or others working in the lab?
- see 1)
- see 1)
- a. Risks to the safety and health of team members or others working in the lab?
- b. Risks to the safety and health of the general public, if released by design or by accident?
- No. All our strains are based on E. coli K12.
- No. All our strains are based on E. coli K12.
- b. Risks to the safety and health of the general public, if released by design or by accident?
- c. Risks to the environment, if released by design or by accident?
- No. All our strains are based on E. coli K12.
- No. All our strains are based on E. coli K12.
- c. Risks to the environment, if released by design or by accident?
- d. Risks to security through malicious misuse by individuals, groups, or countries?
- No. All our strains are based on E. coli K12. All the genes that are introduced are not suited for malicious misuse.
- d. Risks to security through malicious misuse by individuals, groups, or countries?
- 5. If your project moved from a small-scale lab study to become widely used as commercial/industrial product, what new risks might arise? Also, what risks might arise if the knowledge you generate or the methods you develop became widely available?
- If our system become widely used as a commercial/industrial product there doesn't arise new risks. It is only for producing electricity so this scenario has just positive consequences.
- 6. Does your project include any design features to address safety risks?
- Yes it includes a kill switch and an especially safe auxotrophic chassis.
- 7. What safety training have you received (or plan to receive in the future)? Provide a brief description, and a link to your institution’s safety training requirements, if available.
- All the members of our team received instructions by Mr. Dr. Jörn Kalinowski according to German lab safety regulations including working with biological genetically engineered materials.
- All the members of our team received instructions by Mr. Dr. Jörn Kalinowski according to German lab safety regulations including working with biological genetically engineered materials.
- 8. Under what biosafety provisions will / do you work?
- a. Please provide a link to your institution biosafety guidelines.
- Our university has an own health, work and environment protection management system, certified with the British standard [http://uni-bielefeld.agu-hochschulen.de/index.php?id=714 „Occupational Health and Safety Assessment Series“ (BS OHSAS 18001)]
- a. Please provide a link to your institution biosafety guidelines.
Safety forms were approved on October 2nd, 2013 by the iGEM Safety Committee.
 "
"