
Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene)
|
In our search for substances to put in our yoghurt we looked around in articles for molecules with good health effects. From the start we found resveratrol to stand out as a well-studied substance with some huge potential benefits. We mainly consume it via red wine and it is perhaps the main reason for why wine is considered good for your health in reasonable amounts.
Resveratrol belongs to a group of molecules known as Phyloalexin which are used by many plants to battle infections of all sorts.
Since the early 90’s there has been many studies done on resveratrol showing it has a wide range of beneficial properties ranging from skin cancer reduction to anti-inflamatory effects and antioxidant properties.[1]
[1]
Perhaps the most interesting property is that of life extension. With 2.1 billion people being overweight worldwide obesity related diseases are one of humanities greatest health problems today.
Researchers now believe resveratrol is an caloric restricting substance reducing the effect of high caloric food and therefore increasing life expectancy.[2]
|
 |
Methods
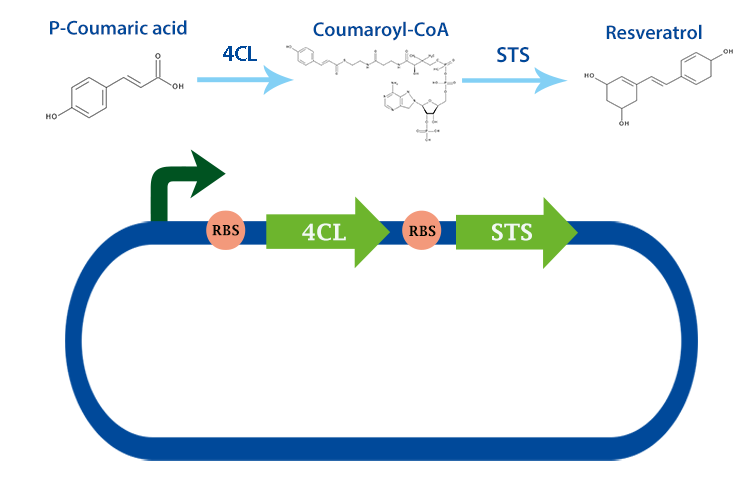
Three enzymes is needed to produce resveratrol. Here we have characterized two of them, 4 coumarate ligase and stilbene synthase. For the first enzyme in the pathway, see the page for tyrosine ammonia lyase.
4-coumarate ligase (4Cl) and stilbene synthase (STS) genes from Arabidophsis thaliana and Vitis vinifiera was obtained from J.Conrado et al.[3]. We biobricked 4Cl and STS with the ribosome binding site B0034 and overhangs in a single PCR. We also made a version with 6-HIS-tag for enzyme expression analysis. After biobricking the two enzymes, we coupled them together to create an operon consisting of 4Cl and STS.
We have expressed 4Cl and STS in E. coli DH5alpha and E. Coli nissle, a probiotic E. coli obtained from Trieste iGEM 2012. 4Cl and STS will also be characterized in lactobacillus, by transforming the construct with our shuttle vector.
Results
Summary
Although we managed to clone out and sequence verify the genes for resveratrol production, we have had some problems in the characterization. The results are unclear, and we did not have time for further investigations.For detailed information about the characterisation methods, see the
protocol section
Biobrick
We succeded in the cloning and sequencing of our two biobricks, 4-Coumarate ligase from
arabidophsis thaliana and Stilbene synthase from vitis vinifiera with the RBS B0034 that should work in various organisms, lactobacillus and E. coli. Sequencing was done at GATC biotech and Uppsala Genome center using sanger sequencing.
We also succeded in the making of our operon containing 4Cl and STS together.
BBa_K1033000 - tyrosine ammonia lyase (TAL) with RBS
BBa_K1033001 - 4 coumarate ligase (4CL) with RBS
BBa_K1033002 - stilbene synthase (STS) with RBS
BBa_K1033003 - B0034-4Cl-B0034-STS
Western blot
We also succeeded in expressing the enzyme stilbene synthase in E. coli. However, our expression of the protein was very weak, and due to time constraints we were not able to optimize our experiment.
To enable the detection of this protein by anti-his antibodies, a 6-histidine tag was incorporated in the sequence. This way we could detect our enzyme with anti-his antibodies.
We expressed our protein with a promotor working in both lactobacillus and E. coli. This way, we can
easily transfer stilbene synthase to lactobacillus later on.
The size of our protein was calculated using ProtParam
[5] to 43 kDA.
 Figure 1: Number 2 shows a very weak band of our protein at around 43 kDA.
Figure 1: Number 2 shows a very weak band of our protein at around 43 kDA.
Positive control -> 1, Stilbene synthase -> 2
High pressure liquid chromatography
We tested our biobrick 4Cl-STS on HPLC, by adding p-coumaric acid as a precursor. The result we saw was quite unclear. We saw that the E. coli produced something out of the ordinary, but the absorbation was low and the peaks did not exactly match the standard. The peak at around ~33 min could correspond to our standard, but it is unclear. We theorize it is something in the actual HPLC measurement that fails, or that something happens to our resveratrol metabolite in our E. coli. This result could correspond to the poor results in our blot. We hope that iGEM teams can continue to work on these biobricks in the future.
4Cl-STS translational fusion expressed in e-coli
 Figure 2: E. coli supposed to produce resveratrol. As we can see, we got very low absorbance peaks at ~30 min, ~33 min and ~36 min.
Resveratrol standard
Figure 2: E. coli supposed to produce resveratrol. As we can see, we got very low absorbance peaks at ~30 min, ~33 min and ~36 min.
Resveratrol standard
 Figure 3: Resveratrol standard, peaks around ~33, ~34 min.
Resveratrol standard scaled
Figure 3: Resveratrol standard, peaks around ~33, ~34 min.
Resveratrol standard scaled
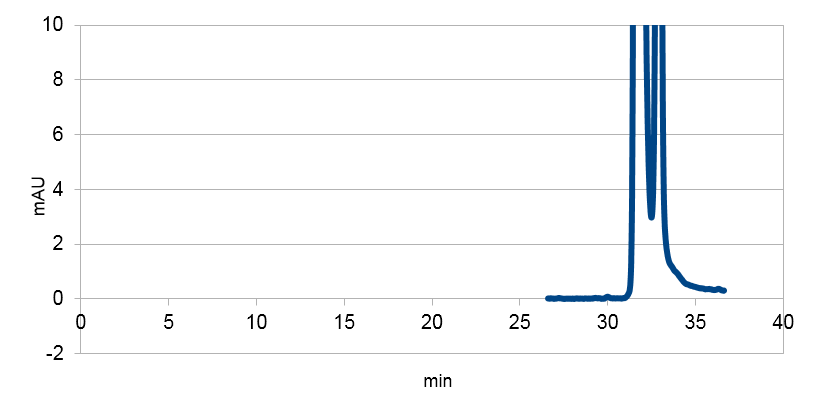 Figure 4: Resveratrol standard that is scaled down to correspond to the absorbations of our e. coli supposed to produce the corresponding metabolite. The peaks are at around ~33 and ~34.
Lysed bacterial culture without plasmid of assembly
Figure 4: Resveratrol standard that is scaled down to correspond to the absorbations of our e. coli supposed to produce the corresponding metabolite. The peaks are at around ~33 and ~34.
Lysed bacterial culture without plasmid of assembly
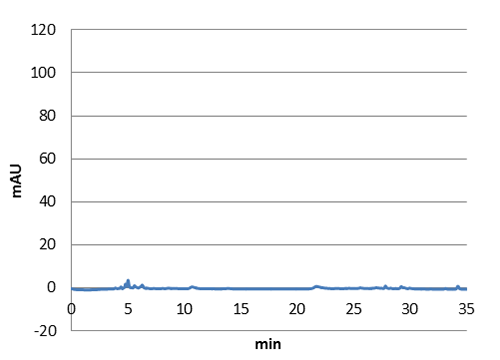 Figure 5: E. coli culture injected to the HPLC without our biobrick tyrosine ammonia lyase. Here we can see that there is originally no peaks around 30-35 minutes.
Figure 5: E. coli culture injected to the HPLC without our biobrick tyrosine ammonia lyase. Here we can see that there is originally no peaks around 30-35 minutes.
References
[1] Sinclair, D. A & Baur, Y, A. Therapeutic potential of resveratrol:
the in vivo evidence. Nature 506 | JUNE 2006 | VOLUME 5
[2] Baur, Y, A. et al. Resveratrol improves health and survival
of mice on a high-calorie diet. Nature Vol 444| 16 November 2006
[3] Robert J. Conrado et al, DNA guided assembly of biosynthetic pathways promotes improved catalytic effiency. Nucleic Acids Research , 2012, Vol 40 NO 4, 1879-1889
[4] Sinclair, D. A & Baur, Y, A. Therapeutic potential of resveratrol:
the in vivo evidence. Nature 506 | JUNE 2006 | VOLUME 5
[5] Baur, Y, A. et al. Resveratrol improves health and survival
of mice on a high-calorie diet. Nature Vol 444| 16 November 2006
[6] Here's a link to ProtParam: http://web.expasy.org/protparam/
 "
"


















