Team:BYU Provo/Notebook/Cholera - Enzyme/October/Period2/Dailylog
From 2013.igem.org
| Cholera - Enzymes Notebook: October 15 - October 31 Daily Log
| ||
|
|
10/15/13 We ran a gel for the colony PCR products we started last night and colony 5 showed a bright band right around 1100 bp's which is the right size for DspB. We then started a 5 ml overnight in LB-Amp from its corresponding streak on the plate.
10/16/13 We ran plasmid purification on our DspB Colony E from the O/N we started yesterday. We then transformed the plasmid into BL21, which is the E. coli strain that we use for expression and purification. The transformation product was plated on LB+AMP and left in the 37° incubator overnight. All procedures were performed according to the protocols listed on our protocols page.
10/17/13 We started a 5 mL overnight of our DspB in BL21 from the plate on 10/16. We also added the AMP to our autoclaved LB. Now we just have to finish waiting for our BL21 to grow up.
10/18/13 We inoculated 500 mL LB+AMP with the 5 mL overnight from yesterday and allowed it to grow for three hours from 4 am to 7 am. At 7 am we added 500 uL IPTG to induce expression of our protein and continued to grow it for another five hours from 7 am to 12 pm. At 12 pm we pelleted the bacteria and placed it in the -80°. We will purify this protein product on Monday.
10/21/13 We purified both our AmyA and DspB protein products using the His-tag purification protocol listed on the protocols page. The protein products were then run on a protein gel and [put results]
10/23/13 We started growing up cholera overnights to run our biofilm degredation assay with our newly purified DspB and AmyA. Samples 1-24 were treated at T = 0 to test for enzymatic inhibition of biofilm growth. Samples 25-48 were treated at T = 48 to test for enzymatic degradation of the biofilm. Treatments were administered according to the following: Biofilm Degradation Overnights 1-3 Control 4-6 Urea 7-9 2.5 uL DspB 10-12 12.5 uL DspB 13-15 25 uL DspB 16-18 2.5 uL AmyA 19-21 12.5 uL AmyA 22-24 25 uL AmyA 25-27 Control 28-30 Urea 31-33 2.5 uL DspB 34-36 12.5 uL DspB 37-39 25 uL DspB 40-42 2.5 uL AmyA 43-45 12.5 uL AmyA 46-48 25 uL AmyA
10/25/13 We began the wash treatments of our Biofilm Assay as outlined in the protocols section. All water washes were performed, excess liquid was removed, and pellets were weighed for pellet size. However, the pellet weight data was inconsistent and unreliable. Pellets were then placed in the freezer over the weekend until Monday.
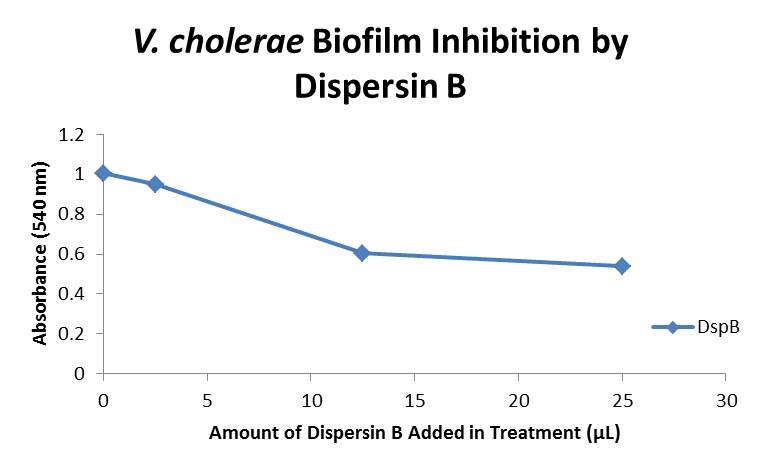
10/28/13 Today we finished the Biofilm Degradation Assay with both our DspB and AmyA enzymes. From this data we graphed the averge absorbance readings for samples treated with 0, 2.5, 12.5, and 25 µL of Dispersin B treated at time T = 0, as this treatment time had the most significant impact on the biofilm growth: There is a distinct reduction in the amount of biofilm growth between untreated and treated samples, with samples treated with 25 µL α-Amylase showing a 40.0% decrease in biofilm formation after 48 hours. While this clearly shows the ability of DspB to inhibit biofilm formation by V. cholerae, we did not see any degradation of the biofilm at treatment T = 48 with one hour of treatment incubation. Further characterization is needed to determine whether AmyA and DspB can degrade the biofilms, and what conditions and concentrations of enzyme would make such degradation possible.
| |
 "
"