Team:Duke/Project/Basics
From 2013.igem.org
Hyunsoo kim (Talk | contribs) (→Background Information) |
Hyunsoo kim (Talk | contribs) (→TAL Effector) |
||
| Line 24: | Line 24: | ||
== TAL Effector == | == TAL Effector == | ||
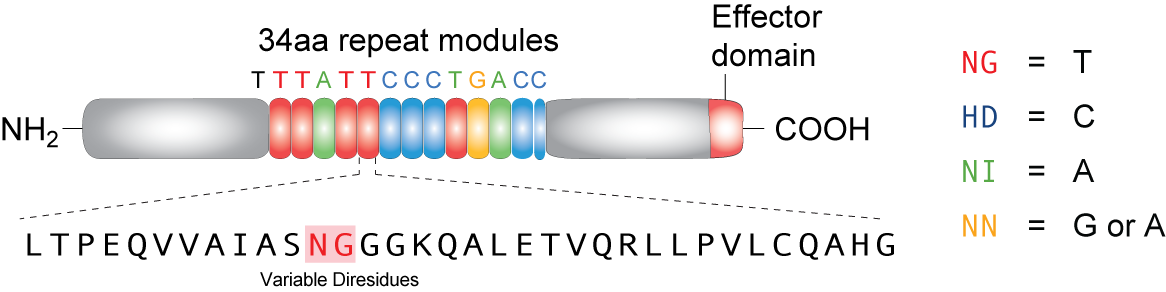
| - | + | TALEs (Transcription Activator-Like effectors or TAL effectors) are DNA binding proteins derived from a plant pathogen <i>Xanthomonas sp.</i> which uses these proteins to regulate the host plant’s genome and aid in bacterial infection. Biochemical analysis of the TALEs revealed a highly repetitive primary structure with two residues at positions 12 and 13 out of a 33-34 amino acid protein that varied based on the target nucleotide. These two amino acids, referred to as repeat variable di-residues (RVDs) can be selected to target any DNA sequence and linked together to bind sequences up to 30 nucleotides long. The four RVDs that we utilize are NG targeting T, HD targeting C, NI targeting A, and NN targeting G, although other RVDs exist that target various nucleotides with varying affinity. Using Golden Gate cloning, we are able to rapidly synthesize TALEs for uses in regulating gene expression by fusing activator or repressor domains to the TALEs. In addition, TALEs have been fused to nuclease domains (TALENs) for applications in genetic engineering and genome editing in plant and mammal cells by editing specific mutations, knocking out genes, or adding genes. We utilize a TALE fused to the fluorescent protein mCherry to act as a transcriptional roadblock rather than a repressor protein with the hopes of achieving the same result. | |
<br> | <br> | ||
[[File:Tale1.png|600px|center|]]<br> | [[File:Tale1.png|600px|center|]]<br> | ||
<div align="center"> Figure 1.1.3. TAL Effector </div> | <div align="center"> Figure 1.1.3. TAL Effector </div> | ||
| - | <br> | + | <br> |
| + | |||
== CRISPR == | == CRISPR == | ||
<br> | <br> | ||
Revision as of 06:06, 27 September 2013
Contents |
Background Information
Genetic Toggle Switch
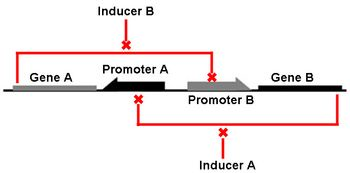
The Genetic Toggle Switch was among the first artificially constructed Gene Regulatory Networks. The original Genetic Toggle Switch consisted of the ptrc-2 (lacI repressible) promoter paired with the temperature sensitive lambda repressor and the PLs1con (lambda repressible) promoter paired with the lacI repressor. Subsequently, induction of the ptrc-2 promoter would repress the PLs1con promoter through expression of the lambda repressor, while induction of the PLs1con promoter would repress the ptrc-2 promoter through expression of the lacI repressor. Switching of the toggle switch from one promoter to another was accomplished by addition of either Isopropyl β-D-1-thiogalactopyranoside (IPTG), which causes the lacI to unbind allowing the ptrc-2 to switch on, or a thermal pulse, which causes the lambda repressor to unbind and allow the PLs1con promoter to switch on. In order to characterize the toggle switch, a GFPmut3 structural gene was placed downstream of the Ptrc-2 promoter so that induction of the Ptrc-2 promoter would result in high expression of GFPmut3 while induction of PLs1con would result in low expression of GFPmut3.
Characteristics of Genetic Toggle Switches
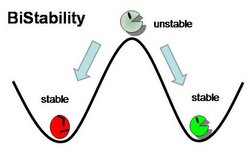
Genetic Toggle Switches have several characteristics which make them unique from other Gene Regulatory networks. For example, Genetic Toggle Switches must be capable of bi-stable behavior, meaning that they should only exist in one of two stable states instead of in a variety of intermediate states. Bi-stability also suggests that a certain threshold must be passed before the toggle switch can switch from one state to another. In addition, toggle switches should have low transcriptional noise to prevent random switching without induction. Finally, a reporter or marker structural gene, such as a fluorescent protein, should be present in order to characterize the effectiveness of the toggle switch. <From: iGEM 2011 Team Duke>
The graph below shows nullclines for the level of two mutually repressive proteins in a bistable toggle switch. Each curve on the graph represents concentrations of lacI and cI that makes its change in concentrations respect to time (ie. d[lacI]/dt = 0, d[cI]/dt=0). These curves are called nullclines, and the points at which the two curves intersect are steady-state solutions of the system because the concentrations of the two repressors do not change with time. However, mathematical analysis shows that the intersection in the middle is an unstable steady state, where minute perturbations can quickly shift the equilibrium point to either direction. On the other hand, the two points labeled “State 1” and “State 2” are stable stead-state solutions. At these two points, the concentration of only one repressor is high while the concentration of the other repressor is minimal. Therefore, with these two stable steady-state solutions, the system is called bistable. Only when the majority repressor is degraded or the minority repressor is over-expressed to “go-over” the separatrix line, the system will flip to the opposite steady state (hence the name bistable toggle switch).
TAL Effector
TALEs (Transcription Activator-Like effectors or TAL effectors) are DNA binding proteins derived from a plant pathogen Xanthomonas sp. which uses these proteins to regulate the host plant’s genome and aid in bacterial infection. Biochemical analysis of the TALEs revealed a highly repetitive primary structure with two residues at positions 12 and 13 out of a 33-34 amino acid protein that varied based on the target nucleotide. These two amino acids, referred to as repeat variable di-residues (RVDs) can be selected to target any DNA sequence and linked together to bind sequences up to 30 nucleotides long. The four RVDs that we utilize are NG targeting T, HD targeting C, NI targeting A, and NN targeting G, although other RVDs exist that target various nucleotides with varying affinity. Using Golden Gate cloning, we are able to rapidly synthesize TALEs for uses in regulating gene expression by fusing activator or repressor domains to the TALEs. In addition, TALEs have been fused to nuclease domains (TALENs) for applications in genetic engineering and genome editing in plant and mammal cells by editing specific mutations, knocking out genes, or adding genes. We utilize a TALE fused to the fluorescent protein mCherry to act as a transcriptional roadblock rather than a repressor protein with the hopes of achieving the same result.
CRISPR
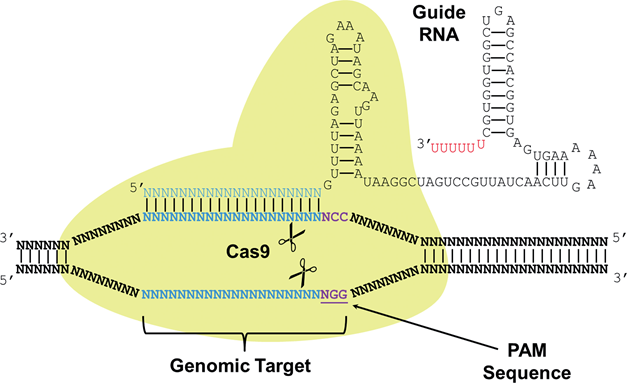
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats, and these are commonly found in archaea (~90%) and in bacteria (~40%). CRISPRs function as bacterial immune response, where it helps recognize foreign DNA and silence it by interacting with CAS (CRISPR-associated) proteins that have nuclease or helicase activity. A small RNA called sgRNA (small-guide RNA) recognize a specific DNA sequence, and its secondary structure forms a region called Cas9 handle, at which the Cas proteins bind to silence the targeted DNA. The recent discovery of CRISPR-CAS system as a tool for genetic engineering and synthetic biology enabled target-specific manipulation of genes. Specifically, by engineering sgRNA sequences that match the target DNA sequences, it forms a complex with Cas protein (Cas9, having nuclease activity) to cut the target gene. Also, by using mutated nuclease-deficient Cas9 protein (dCas9), engineering site-specific proteins that can act as transcription factors (repressors) is possible.
References
DiCarlo et al., 2013
- Gardener, T. et al. Construction of a genetic toggle switch in Escherichia coli. Nature. 403, 339-342 (2000).
- TAL Effectors Resources. Introduction to TAL Effectors. Accessd on 9/3/13 <http://www.genome-engineering.org/taleffectors/>.
- DiCarlo, J. et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Research. 41(7), 4336-4343 (2013).
 "
"