Team:Paris Saclay/Notebook/August/22
From 2013.igem.org
| Line 33: | Line 33: | ||
* H2O : 6 µL | * H2O : 6 µL | ||
| - | We let digestions at 37°C during 10 minutes | + | We let digestions at 37°C during 10 minutes. |
| - | + | ===='''Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in PSB3K3'''==== | |
| - | + | ||
| - | + | ||
===='''1 - Gel purification of the digestion of Bba_J04450 by EcoRI/PstI '''==== | ===='''1 - Gel purification of the digestion of Bba_J04450 by EcoRI/PstI '''==== | ||
| Line 52: | Line 50: | ||
The Nanodrop gives us a very few quantity of PSB3K3 so we decided to check it with a first electrophoresis. | The Nanodrop gives us a very few quantity of PSB3K3 so we decided to check it with a first electrophoresis. | ||
|} | |} | ||
| + | |||
| + | ===='''2 - Electrophoresis of gel purification of the digestion of Bba_J04450 by EcoRI/PstI '''==== | ||
| + | |||
| + | XiaoJing | ||
{| | {| | ||
| Line 66: | Line 68: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We can see anything with the fisrt electrophoresis that's why we made an EtOH precipitation. | + | We can't see anything with the fisrt electrophoresis that's why we made an EtOH precipitation. |
|} | |} | ||
| + | |||
| + | ===='''3 - Ethanol precipitation of the digestion of Bba_J04450 by EcoRI/PstI '''==== | ||
Protocol : [[Team:Paris_Saclay/ethanol|EtOH precipitation]] | Protocol : [[Team:Paris_Saclay/ethanol|EtOH precipitation]] | ||
| - | We used 34µL of DNA. | + | We used 34µL of DNA. |
| + | |||
==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== | ==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== | ||
| Line 124: | Line 129: | ||
* RBS-BphR2 Part I, tube 1 : 42ng/µL | * RBS-BphR2 Part I, tube 1 : 42ng/µL | ||
* RBS-BphR2 Part I, tube 2 : 75ng/µL | * RBS-BphR2 Part I, tube 2 : 75ng/µL | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 18:22, 28 September 2013
Notebook : August 22
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
1 - Plasmid extraction of Bba_K1155000 from DH5α
Nguyen
Protocol : High copy plamid extraction
Nanodrop :
- Bba_K1155000 : 175ng/µL
|
The extraction was good. We will digested the plasmid. |
2 - Digestion of Bba_K1155000 by SpeI
Nguyen
Used quantities :
- Bba_K1155000 : 10µL
- Buffer FD : 2µL
- Spe I : 2µL
- H2O : 6 µL
We let digestions at 37°C during 10 minutes.
Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in PSB3K3
1 - Gel purification of the digestion of Bba_J04450 by EcoRI/PstI
XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- PSB3K3 : 4ng/µL
|
The Nanodrop gives us a very few quantity of PSB3K3 so we decided to check it with a first electrophoresis. |
2 - Electrophoresis of gel purification of the digestion of Bba_J04450 by EcoRI/PstI
XiaoJing
| ]] |
|
Expect sizes :
- PSB3K3 : 2750 bp
|
We can't see anything with the fisrt electrophoresis that's why we made an EtOH precipitation. |
3 - Ethanol precipitation of the digestion of Bba_J04450 by EcoRI/PstI
Protocol : EtOH precipitation
We used 34µL of DNA.
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining BphR2 protein
1 - PCR of BphR2 Part I
Damir
Used quantities :
- Oligo 54F : 2µL
- Oligo 55R : 2µL
- DNA : 1µL
- Buffer Phusion : 10µL
- dNTP : 1µL
- Phusion : 1µL
- DMS9 : 2µL ??????????????????????????????????
- H2O : 31µL
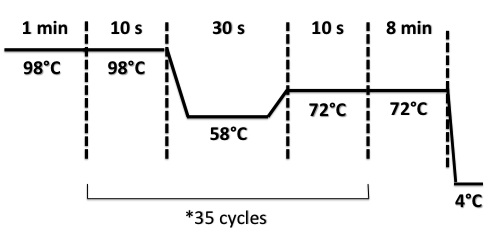
PCR program :
2 - Electrophoresis of PCR product : BphR2 Part I
Damir
| ]] |
|
Expected sizes : Bphr2 Part I : 178 kb
|
We obtain fragments at the right size. We can purify it. |
3 - Gel purification of PCR product : BphR2 Part I
Damir
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- RBS-BphR2 Part I, tube 1 : 42ng/µL
- RBS-BphR2 Part I, tube 2 : 75ng/µL
| Previous day | Back to calendar | Next day |
 "
"