Team:Warsaw/Project description
From 2013.igem.org
(→FluoSafe: acrylamide sensor) |
m (→FluoSafe: acrylamide sensor) |
||
| Line 26: | Line 26: | ||
Such standardized 'BiFC toolbox' (with measured sensitivity and specificity for each individual combination of fragments) will be an important contribution to the Registry of Standard Biological Parts. | Such standardized 'BiFC toolbox' (with measured sensitivity and specificity for each individual combination of fragments) will be an important contribution to the Registry of Standard Biological Parts. | ||
| - | In parallel, our second goal is to develop an alternative acrylamide sensor based on redox state reporter – the roGFP protein.The roGFP protein fused with human gluteredoxin is a highly-specific sensor of the cellular glutathione redox potential (glutathione is a simple tripeptide which takes part e.g. in the cell detoxication process | + | In parallel, our second goal is to develop an alternative acrylamide sensor based on redox state reporter – the roGFP protein.The roGFP protein fused with human gluteredoxin is a highly-specific sensor of the cellular glutathione redox potential (glutathione is a simple tripeptide which takes part e.g. in the cell detoxication process). |
Revision as of 09:37, 29 September 2013
Project description
FluoSafe: acrylamide sensor
This year Warsaw iGEM team is presenting to you FluoSafe - a bacterial sensor for acrylamide detection. As many of you know acrylamide is usually present in biochemical laboratories. But what is the significance of this organic compound really? Well, it is a neurotoxin and its carcinogenic activity has been widely proven.
Not only is it present in our work environment but also in our food. You might think that if something is not mentioned on the food label it means that that substance cannot be found in what you eat. Well - unfortunately this is not true. Acrylamide is found in chips, french fries, and anything that contains amino acids, reducing sugar and is subjected to heat. This environment is perfect for the [http://en.wikipedia.org/wiki/Maillard_reaction Maillard reaction] to occur. Once this reaction has taken place, we can find acrylamide in our food.
This is the reason why our project is focusing on detecting acrylamide not only in laboratories all over the world, but also in the food we eat. Our aim is also to create a sensor that can be widely used and is cheap enought to be more available for anyone who would like to use it.
As it has been demonstrated before acrylamide reacts with the C-terminal valine of both hemoglobin subunits, creating adducts. When this reaction has occurred, it is possible to measure acrylamide level by using ion selective electrode coated with human hemoglobin and then observing the change in current intensity to voltage ratio.
The aim of the current project is to create a bacterial sensor using an E. coli strain which can produce functional human hemoglobin (this idea is based on the system designed in 2007 by the Berkeley iGEM Team).
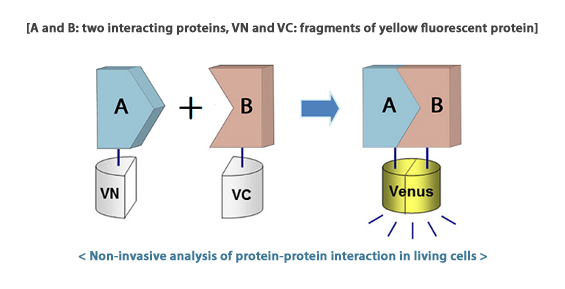
We plan to implement [http://en.wikipedia.org/wiki/BiFC bimolecular fluorescence complementation (BiFC)] to our system. In order to do this we will create a fusion protein composed of α and β hemoglobin subunits with complementary parts of split sfGFP ( an improved superfolder green fluorescent protein derivative, which is often used as a marker in molecular biology). When there is no acrylamide in the environment both chains of hemoglobin form a stabile complex allowing to reconstruct a functional, fluorescent protein.
On the other hand, formation of acrylamide adducts at the C-end of hemoglobin chains reduce their mutual affinity, so they are unable to bring the split parts of sfGFP within proximity and fluorescence complementation cannot occur. This should lead to an observable decrease in fluorescence intensity in cells exposed to acrylamide.
We aim to create and test a variety of constructs that will allow an implementation of different BiFC variations. We are planning to use some already applied fluorescent proteins (but unavailable in the BioBrick format): eGFP, sfGFP and mCherry. Additionally, we intend to create constructs based on proteins that have not been applied yet such as: sfCFP, sfBFP, sfYFP, sfVenus i sfCerulean (improved GFP derivatives for BiFC, with additional superfolder mutations). We also intend to compare the above-mentioned combinations to the already available Venus- and Cerulean-based systems.
Such standardized 'BiFC toolbox' (with measured sensitivity and specificity for each individual combination of fragments) will be an important contribution to the Registry of Standard Biological Parts.
In parallel, our second goal is to develop an alternative acrylamide sensor based on redox state reporter – the roGFP protein.The roGFP protein fused with human gluteredoxin is a highly-specific sensor of the cellular glutathione redox potential (glutathione is a simple tripeptide which takes part e.g. in the cell detoxication process).
It should be emphasized that in this method the redox state change does not lead to a decrease in the level of fluorescence, but actually causes a shift in the excitation maximum, thus making the outcomes more noticeable. The typical way of acrylamide detoxication in a cell involves conjugating acrylamide to glutathione by S-glutathione transpherase (Cui. S et al.). Thus, the influence of acrylamide on cellular glutathione pool can be easily applied for detecting its presence and concentration changes in tested solution.
Our final goal is not only to construct our sensor but also to examine the cytotoxicity caused by acrylamide exposure. Our aim is to show how acrylamide may affect different tissues. This part of our project is going to demonstrate how different cell lines (i.e derived from different tissues) react to acrylamide exposure.
Bibliography:
- Krajewska, A., Radecki, J. & Radecka, H. A Voltammetric Biosensor Based on Glassy Carbon Electrodes Modified with Single-Walled Carbon Nanotubes/Hemoglobin for Detection of Acrylamide in Water Extracts from Potato Crisps. Sensors 8, 5832–5844 (2008).
- UC Berkeley 2007 iGEM project ([1])
- Zhou, J., Lin, J. & Zhou, C. An improved bimolecular fluorescence complementation tool based on superfolder green fluorescent protein. Acta biochimica et … 43, 239–244 (2011).
- Pédelacq, J.-D., Cabantous, S., Tran, T., Terwilliger, T. C. & Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nature biotechnology 24, 79–88 (2006).
- Kodama, Y. & Hu, C.-D. Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. BioTechniques 53, 285–98 (2012).
- Hanson, G. T. et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. The Journal of biological chemistry 279, 13044–53 (2004).
- Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nature methods 5, 553–9 (2008).
- Cui, S., Kim, S., Jo, S. & Lee, Y. A study of in vitro scavenging reactions of acrylamide with glutathione using electrospray ionization tandem mass spectrometry. BULLETIN-KOREAN … (2005).at [http://newjournal.kcsnet.or.kr/main/j_search/j_download.htm?code=B050816]
 "
"