Team:Bielefeld-Germany/Project/GldA
From 2013.igem.org
| Line 111: | Line 111: | ||
| - | *<p align="justify">[[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)|SDS-PAGE]] shows protein Glycerol dehydrogenase at expected size of 40 kDa. In contrast to ''Escherichia coli'' KRX | + | *<p align="justify">[[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)|SDS-PAGE]] shows protein Glycerol dehydrogenase at expected size of 40 kDa. In contrast to ''Escherichia coli'' KRX wild type, weak Anderson promoter (<bbpart>BBa_K1172205</bbpart>) shows only a slightly stronger band, whereas T7 (<bbpart>BBa_K1172203</bbpart>) and Lac (<bbpart>BBa_K1172204</bbpart>) promoter show a strong band, which is equated with a strong expression and overproduction of GldA.</p> |
| Line 127: | Line 127: | ||
| - | *<p align="justify">We could observe an enhanced NADH production for ''Escherichia coli'' KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172205</bbpart> with increasing promoter strength and in comparison with ''Escherichia coli'' KRX | + | *<p align="justify">We could observe an enhanced NADH production for ''Escherichia coli'' KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172205</bbpart> with increasing promoter strength and in comparison with ''Escherichia coli'' KRX wild type. (Table 2 and Figure 5)</p> |
| - | [[Image:IGEM_Bielefeld_Table6_Results_NADH.jpg|300px|thumb|left|<p align="justify"> '''Table 2: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Table6_Results_NADH.jpg|300px|thumb|left|<p align="justify"> '''Table 2: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart>. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_Figure9_NADH.jpg|300px|thumb|center|<p align="justify"> '''Figure 5: Column Chart with results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Figure9_NADH.jpg|300px|thumb|center|<p align="justify"> '''Figure 5: Column Chart with results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX wild typ and ''Escherichia coli'' KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart>. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.'''</p>]] |
| - | *<p align="justify">Compared with ''Escherichia coli'' KRX | + | *<p align="justify">Compared with ''Escherichia coli'' KRX wild type, ''Escherichia coli'' with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) shows 1,7 μM NADH overproduction and GldA with Lac promoter (<bbpart>BBa_K1172204</bbpart>) shows 0,2 μM NADH overproduction. The GldA expression by the Anderson promoter (<bbpart>BBa_K1172205</bbpart>) is too weak for an efficient NADH overproduction.</p> |
*<p align="justify">Thus, ''E. coli'' with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) is used for all further tests, because this strain shows by far the best NADH production rate.</p> | *<p align="justify">Thus, ''E. coli'' with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) is used for all further tests, because this strain shows by far the best NADH production rate.</p> | ||
| Line 144: | Line 144: | ||
| - | *<p align="justify">''E.coli'' grown in glycerol supplemented medium shows great enhanced NADH production in contrast to ''Escherichia coli'' KRX | + | *<p align="justify">''E.coli'' grown in glycerol supplemented medium shows great enhanced NADH production in contrast to ''Escherichia coli'' KRX wild type and also in contrast to'' Escherichia coli'' KRX with <bbpart>BBa_K1172203</bbpart> and no further NADH supplementation. (Table 4 and Figure 6)</p> |
| - | [[Image:IGEM_Bielefeld_Table8_Results_NADH.jpg|300px|thumb|left|<p align="justify"> '''Table 4: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Table8_Results_NADH.jpg|300px|thumb|left|<p align="justify"> '''Table 4: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172203</bbpart> supplemented with different amounts of glycerol to [[Team:Bielefeld-Germany/Labjournal/Molecular#LB medium|LB medium]]. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_Figure10_Column_NADH.jpg|300px|thumb|center|<p align="justify"> '''Figure 6: Column Chart with results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between'' Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Figure10_Column_NADH.jpg|300px|thumb|center|<p align="justify"> '''Figure 6: Column Chart with results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between'' Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172203</bbpart> supplemented with different amounts of glycerol to [[Team:Bielefeld-Germany/Labjournal/Molecular#LB medium|LB medium]]. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | *<p align="justify">Glycerol dependent [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]] shows a quiet good NADH overproduction for <bbpart>BBa_K1172203</bbpart> with supplementation of glycerol. It was possible to increase NADH production up to 270 % in comparison to ''Escherichia coli'' KRX | + | *<p align="justify">Glycerol dependent [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]] shows a quiet good NADH overproduction for <bbpart>BBa_K1172203</bbpart> with supplementation of glycerol. It was possible to increase NADH production up to 270 % in comparison to ''Escherichia coli'' KRX wild type and up to 100 % in comparison to ''Escherichia coli'' KRX with <bbpart>BBa_K1172203</bbpart> and without glycerol supplementation. However, it is relatively unimportant how much glycerol is supplemented to the medium. However, this growth characteristics is initially only valid for [[Team:Bielefeld-Germany/Labjournal/Molecular#LB medium|LB medium]] glycerol supplementation. </p> |
| - | *<p align="justify">With former [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assays]] we only observed the effects of GldA on the intracellular NADH concentration of ''E. coli''. To investigate additionally extracellular NADH concentration, we tested ''Escherichia coli'' KRX | + | *<p align="justify">With former [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assays]] we only observed the effects of GldA on the intracellular NADH concentration of ''E. coli''. To investigate additionally extracellular NADH concentration, we tested ''Escherichia coli'' KRX wild type strain and ''E. coli'' KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart> by cultivation with different glycerol concentrations in [[Team:Bielefeld-Germany/Labjournal/Molecular#M9 minimal medium|M9 medium]]. (Table 5)</p> |
| Line 160: | Line 160: | ||
| - | *<p align="justify">Cultivating ''Escherichia coli'' <bbpart>BBa_K1172203</bbpart> with increasing glycerol concentration of [[Team:Bielefeld-Germany/Labjournal/Molecular#M9 minimal medium | M9 medium]] shows enhanced intracellular and also enhanced extracellular NADH concentration in contrast to ''Escherichia coli'' KRX | + | *<p align="justify">Cultivating ''Escherichia coli'' <bbpart>BBa_K1172203</bbpart> with increasing glycerol concentration of [[Team:Bielefeld-Germany/Labjournal/Molecular#M9 minimal medium | M9 medium]] shows enhanced intracellular and also enhanced extracellular NADH concentration in contrast to ''Escherichia coli'' KRX wild type. (Table 6 and Figure 7)</p> |
| - | [[Image:IGEM_Bielefeld_Table10_Results_NADH.jpg|300px|thumb|left|<p align="justify"> '''Table 6: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Table10_Results_NADH.jpg|300px|thumb|left|<p align="justify"> '''Table 6: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172203</bbpart> grown in [[Team:Bielefeld-Germany/Labjournal/Molecular#M9 minimal medium | M9 medium]] with different concentrations of glycerol. NADH concentration with standard deviation and concentration in comparison to the wild type is shown for cell disruption and supernatant fraction. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_Figure11_Column_NADHAssay.jpg|300px|thumb|center|<p align="justify"> '''Figure 7: Column Chart with results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Figure11_Column_NADHAssay.jpg|300px|thumb|center|<p align="justify"> '''Figure 7: Column Chart with results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NADH-Assay|NADH-assay]]. Comparison between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172203</bbpart> grown in [[Team:Bielefeld-Germany/Labjournal/Molecular#M9 minimal medium | M9 medium]] with different concentrations of glycerol. NADH concentration with standard deviation and concentration in comparison to the wild type is shown for cell disruption (Intracellular) and supernatant (Extracellular) fraction. Two biological and at least three technical replicates were analyzed. '''</p>]] |
*<p align="justify">In addition to previously observed increase in intracellular NADH concentration, we were able to show an enhanced extracellular NADH concentration. Heterologous expression of GldA <bbpart>BBa_K1172203</bbpart> in ''E. coli'' seems to be an appropriate way for endogenous mediator production. Furthermore glycerol could be confirmed as a suitable substrate for the NADH production. </p> | *<p align="justify">In addition to previously observed increase in intracellular NADH concentration, we were able to show an enhanced extracellular NADH concentration. Heterologous expression of GldA <bbpart>BBa_K1172203</bbpart> in ''E. coli'' seems to be an appropriate way for endogenous mediator production. Furthermore glycerol could be confirmed as a suitable substrate for the NADH production. </p> | ||
| - | *<p align="justify">Intracellular NADH concentration increases up to 223 % in comparison to ''Escherichia coli'' KRX | + | *<p align="justify">Intracellular NADH concentration increases up to 223 % in comparison to ''Escherichia coli'' KRX wild type with increasing promoter strength. Besides it is very interesting to see that extracellular NADH concentration is 7 times higher than the intracellular concentration for all strains. Heterologous expression of GldA <bbpart>BBa_K1172203</bbpart> in ''E. coli'' causes a 2.5 times higher extracellular NADH concentration compared with ''Escherichia coli'' KRX wild type. </p> |
*<p align="justify">These data show that NADH is an adequate mediator for Microbial Fuel Cells. NADH can be transported across the cell membrane which is indicated by much higher extracellular NADH concentration and thus allow NADH-mediated electron transfer (EET).</p> | *<p align="justify">These data show that NADH is an adequate mediator for Microbial Fuel Cells. NADH can be transported across the cell membrane which is indicated by much higher extracellular NADH concentration and thus allow NADH-mediated electron transfer (EET).</p> | ||
| Line 176: | Line 176: | ||
*<p align="justify">All NADH-assays showed great enhanced NADH overproduction for GldA strain. Therefore higher electron transport efficiency should result in an improved bioelectricity output.</p> | *<p align="justify">All NADH-assays showed great enhanced NADH overproduction for GldA strain. Therefore higher electron transport efficiency should result in an improved bioelectricity output.</p> | ||
| - | *<p align="justify">For testing the genetic engineered system in the Microbial Fuel Cell, we used ''Escherichia coli'' KRX with GldA and Lac promoter (<bbpart>BBa_K1172204</bbpart>) in contrast to ''Escherichia coli'' KRX | + | *<p align="justify">For testing the genetic engineered system in the Microbial Fuel Cell, we used ''Escherichia coli'' KRX with GldA and Lac promoter (<bbpart>BBa_K1172204</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Certainly NADH-assays determined ''Escherichia coli'' KRX with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) as the best endogenous mediator producing strain. Unfortunately we could not use this strain due to cultivation problems. </p> |
*<p align="justify">According to our assumptions, the extracellular electron transfer mediated by NADH is improved in the GldA strain resulting in an increased bioelectricity output. (Figure 8 and 9)</p> | *<p align="justify">According to our assumptions, the extracellular electron transfer mediated by NADH is improved in the GldA strain resulting in an increased bioelectricity output. (Figure 8 and 9)</p> | ||
| - | [[Image:IGEM_Bielefeld_Voltage_GldA_Genetic.jpg|300px|thumb|left|<p align="justify"> '''Figure 8: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) in contrast to ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_Voltage_GldA_Genetic.jpg|300px|thumb|left|<p align="justify"> '''Figure 8: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Voltage curve from ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) is shown over time over a resistance of 200 Ω. M9-medium was used with no supplementation of mediators. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_ElectricCharge2_GldA_Genetic.jpg|300px|thumb|center|<p align="justify"> '''Figure 9: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) in contrast to ''Escherichia coli'' KRX | + | [[Image:IGEM_Bielefeld_ElectricCharge2_GldA_Genetic.jpg|300px|thumb|center|<p align="justify"> '''Figure 9: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Electric charge curve from ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) is shown over time over a resistance of 200 Ω. M9-medium was used with no supplementation of mediators. '''</p>]] |
| - | *<p align="justify">''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) shows 27 % higher maximal voltage than ''Escherichia coli'' KRX | + | *<p align="justify">''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>) shows 27 % higher maximal voltage than ''Escherichia coli'' KRX wild type. Over the whole cultivation, voltage was about 20% improved. The calculation of the electric charge confirms the described results. Electric charge is equivalent to the number of transported electrons and 20 % enhanced for ''Escherichia coli'' KRX with GldA (<bbpart>BBa_K1172204</bbpart>). The maximal electric charge of 135 mC examines that overexpression of glycerol dehydrogenase enables endogenous mediator production and more efficient electron shuttle-mediated electron transfer. </p> |
*<p align="justify">A further optimization by using ''Escherichia coli'' KRX with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) instead of Lac promoter (<bbpart>BBa_K1172203</bbpart>) would improve electricity output according NADH-assay results (Table 2 and Figure 5) of about 134 %. These results demonstrate that the redox mediator NADH from ''Escherichia coli'' facilitates the electron transfer between cell and electrode and shows a starting point for reducing costs by expensive and toxix exogenous mediators.</p> | *<p align="justify">A further optimization by using ''Escherichia coli'' KRX with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) instead of Lac promoter (<bbpart>BBa_K1172203</bbpart>) would improve electricity output according NADH-assay results (Table 2 and Figure 5) of about 134 %. These results demonstrate that the redox mediator NADH from ''Escherichia coli'' facilitates the electron transfer between cell and electrode and shows a starting point for reducing costs by expensive and toxix exogenous mediators.</p> | ||
| Line 192: | Line 192: | ||
*<p align="justify">Mediators are essential for the use of ''Escherichia coli'' in Microbial Fuel Cells. The main advantage of improving MFCs is to enhance kinetics of the electron transfer between the bacterial cells and the fuel cell anode. In order to decrease the usage of expensive and toxic synthetic mediators (exogenous mediators like methylene blue oder neutral red), we produced the endogenous mediator NADH by overexpression of glycerol dehydrogenase. </p> | *<p align="justify">Mediators are essential for the use of ''Escherichia coli'' in Microbial Fuel Cells. The main advantage of improving MFCs is to enhance kinetics of the electron transfer between the bacterial cells and the fuel cell anode. In order to decrease the usage of expensive and toxic synthetic mediators (exogenous mediators like methylene blue oder neutral red), we produced the endogenous mediator NADH by overexpression of glycerol dehydrogenase. </p> | ||
| - | *<p align="justify">Looking at our project in its entirety, the overproduction of glycerol dehydrogenase seems to be a great way for endogenous mediator production. The use of glycerol as our main carbon source for ''Escherichia coli'' in the MFC further enhances the efficiency of NADH production. We can show a 270 % higher intracellular NADH concentration (4 μM) for ''Escherichia coli'' KRX with GldA plasmid and 170 % higher extracellular NADH concentration (24 μM) in contrast to ''Escherichia coli'' KRX | + | *<p align="justify">Looking at our project in its entirety, the overproduction of glycerol dehydrogenase seems to be a great way for endogenous mediator production. The use of glycerol as our main carbon source for ''Escherichia coli'' in the MFC further enhances the efficiency of NADH production. We can show a 270 % higher intracellular NADH concentration (4 μM) for ''Escherichia coli'' KRX with GldA plasmid and 170 % higher extracellular NADH concentration (24 μM) in contrast to ''Escherichia coli'' KRX wild type.</p> |
*<p align="justify">The heterologous expression of GldA is a great genetic strategy to optimize mediator production as well as electricity generation in Microbial Fuel Cells. The most suitable and efficient GldA device for ''Escherichia coli'' is a combination with Rhamnose inducible T7 promoter (<bbpart>BBa_K1172203</bbpart>).</p> | *<p align="justify">The heterologous expression of GldA is a great genetic strategy to optimize mediator production as well as electricity generation in Microbial Fuel Cells. The most suitable and efficient GldA device for ''Escherichia coli'' is a combination with Rhamnose inducible T7 promoter (<bbpart>BBa_K1172203</bbpart>).</p> | ||
Revision as of 14:29, 4 October 2013
GldA
Glycerol dehydrogenase GldA - Overview
Mediators are essential for the use of Escherichia coli in Microbial Fuel Cells. The main advantage of improving MFCs is to enhance kinetics of the electron transfer between the bacterial cells and the fuel cell anode. Enhancing the mediator concentration in the MFC is an efficient way for higher electron transfer. In order to decrease the usage of expensive and toxic synthetic mediators, we engineered an E. coli KRX strain with overexpression of glycerol dehydrogenase (GldA). GldA produces the endogenous mediator NADH from NAD+ and glycerol, which is the main carbon source of our medium. Efficient mediators were produced by the optimized E. coli. We demonstrate that engineering E. coli by introduction of appropriate oxidoreductase via gene manipulation can greatly improve the mediator production and power generation. We can show an extremely increased intracellular- and extracellular NADH concentration. This leads to 20 % enhanced current production in our Microbial Fuel Cell. The overexpression of Glycerol dehydrogenase from Escherichia coli is a great genetic optimization for electron shuttle-mediated extracellular electron transfer from bacteria to electrodes.
Theory
Escherichia coli is a readily available and easily grown bacterium and has become a popular biocatalyst used in MFCs in spite of no conductivity by nature (Park et al., 2000). On way of transferring electrons generated from redox reactions inside E. coli cells to the anode is using mediators. Mediators are shuttling the electrons from inside of the bacterial cell to the anode. There are several drawbacks to using exogenous mediators (e.g. neutral red or methylene blue), such as their expense, short lifetime, and toxicity to the microorganisms (Seopet al., 2006). However, when the bacteria produce their own mediators the system operates at a high, sustained level of activity. Such a system is called mediator-less Microbial Fuel Cell, because exogenous mediators do not need to be added. Thus they are of great importance in MFC applications.
To significantly improve the MFC performance, a large amount of the bacteria-excreted mediators must be generated. This is a great challenge because of high metabolic stress for the cell (Qiao et al., 2008).
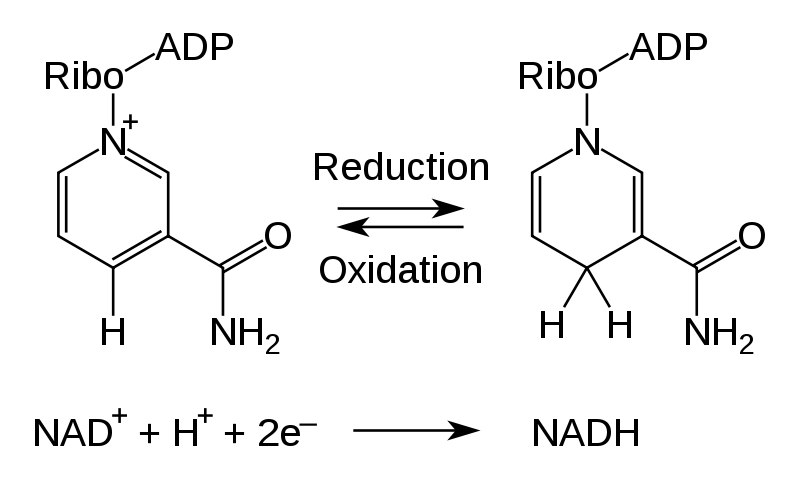
Therefore our plan was it to overexpress glycerol dehydrogenase (GldA) from Escherichia coli in order to minimize the metabolic treatment which would be caused by genes foreign to the species. Most of the GldA genetic products are small water-soluble redox molecules, which have properties similar to mediators (Kelley and Dekker, 1985). GldA has a broad substrate specificity with involvement in the metabolism of different pathways. The main function of this oxidoreductase (EC 1.1.1.6) is the catalyzation of the chemical reaction Glycerol + NAD+ --> Glycerone + NADH + H+ with NADH as the main endogenous mediator.
NAD+ is reduced to NADH by addition of two electrons (e-) and one proton (H+) and therefore very suitable as a electron mediator. (Ruzheinikov et al., 2001)
Overexpression of glycerol dehydrogenase GldA from Escherichia coli will enhance the amount of mediator in the MFC causing improved electron shuttle-mediated extracellular electron transfer and finally more efficient electricity generation by the microorganisms.
Genetic Approach
The GldA gene from Escherichia coli was cloned and heterologously expressed in E. coli KRX under the control of different promoters (Table 1).

Figure 3: pSB1C3 – <bbpart>BBa_K1172201</bbpart> GldA BioBrick (1104 bp) was examined by restriction analysis and sequencing.
Results
Upon the expression of the glycerol dehydrogenase, the endogenous mediator production of Escherichia coli was measured. SDS-PAGE combined with MALDI-TOF MS/MS and different NADH-assays characterize GldA BioBrick <bbpart>BBa_K1172201</bbpart>.
SDS-PAGE and MALDI-TOF
SDS-PAGE shows protein Glycerol dehydrogenase at expected size of 40 kDa. In contrast to Escherichia coli KRX wild type, weak Anderson promoter (<bbpart>BBa_K1172205</bbpart>) shows only a slightly stronger band, whereas T7 (<bbpart>BBa_K1172203</bbpart>) and Lac (<bbpart>BBa_K1172204</bbpart>) promoter show a strong band, which is equated with a strong expression and overproduction of GldA.

Figure 4: SDS-PAGE with [http://www.thermoscientific.com/ecomm/servlet/productsdetail_11152___13576050_-1 Prestained Protein Ladder from Thermo Scientific] as marker. Comparison of protein expression between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart> after total cell disruption. The right-hand gel was loaded with a higher protein concentration. SDS-PAGE shows protein Glycerol dehydrogenase at expected size of 40 kDa. Enhanced overproduction with increasing promoter strength.
Furthermore we were able to identify the overexpressed glycerol dehydrogenase (Figure. 4) with MALDI-TOF MS/MS.
Tryptic digest of the gel lane for analysis with MALDI-TOF could examine the glycerol dehydrogenase with a Mascot Score of 266 against Escherichia coli database.
NADH-Assays
An overproduction of the glycerol dehydrogenase results of course in an overproduction of products from glycerol dehydrogenase. Glycerol dehydrogenase produces several mediators because of its broad substrate specificity. GldA is involved in several metabolism pathways, for example in the glycerol metabolism, which converts glycerol and NAD+ to Glycerone, NADH and H+. NADH is a small, water-soluble redoxmolecule, which seems to be a great mediator for Microbial Fuel Cells. An overexpression of glycerol dehydrogenase leads to an overexpression of NADH and therefore to an endogenous mediator production. Furthermore GldA seems to be very interesting in combination with our preferred MFC carbon source glycerol. Thus, it is essential to get reliable data on NADH overproduction to see how many electron shuttles can be available and how efficient electron shuttle-mediated electron transfer (EET) will be.
We could observe an enhanced NADH production for Escherichia coli KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172205</bbpart> with increasing promoter strength and in comparison with Escherichia coli KRX wild type. (Table 2 and Figure 5)
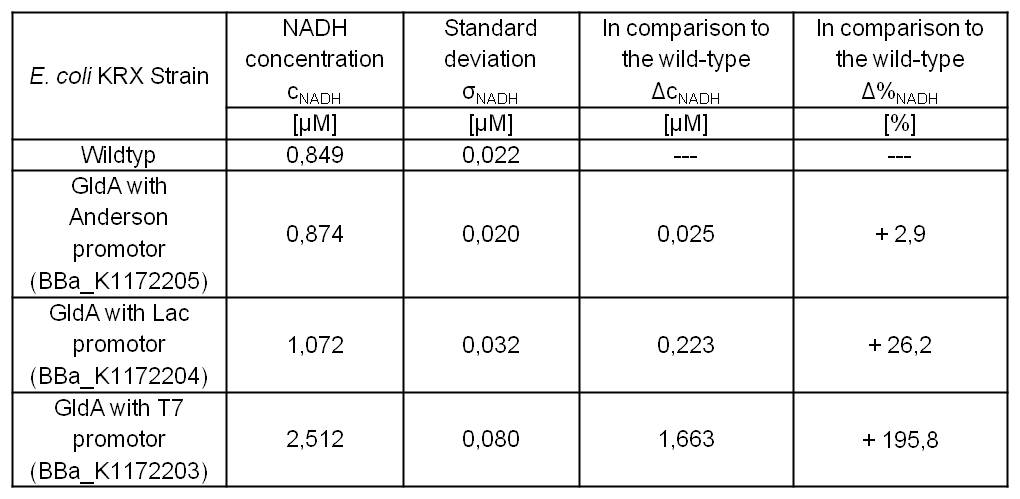
Table 2: Results of the NADH-assay. Comparison between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart>. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.
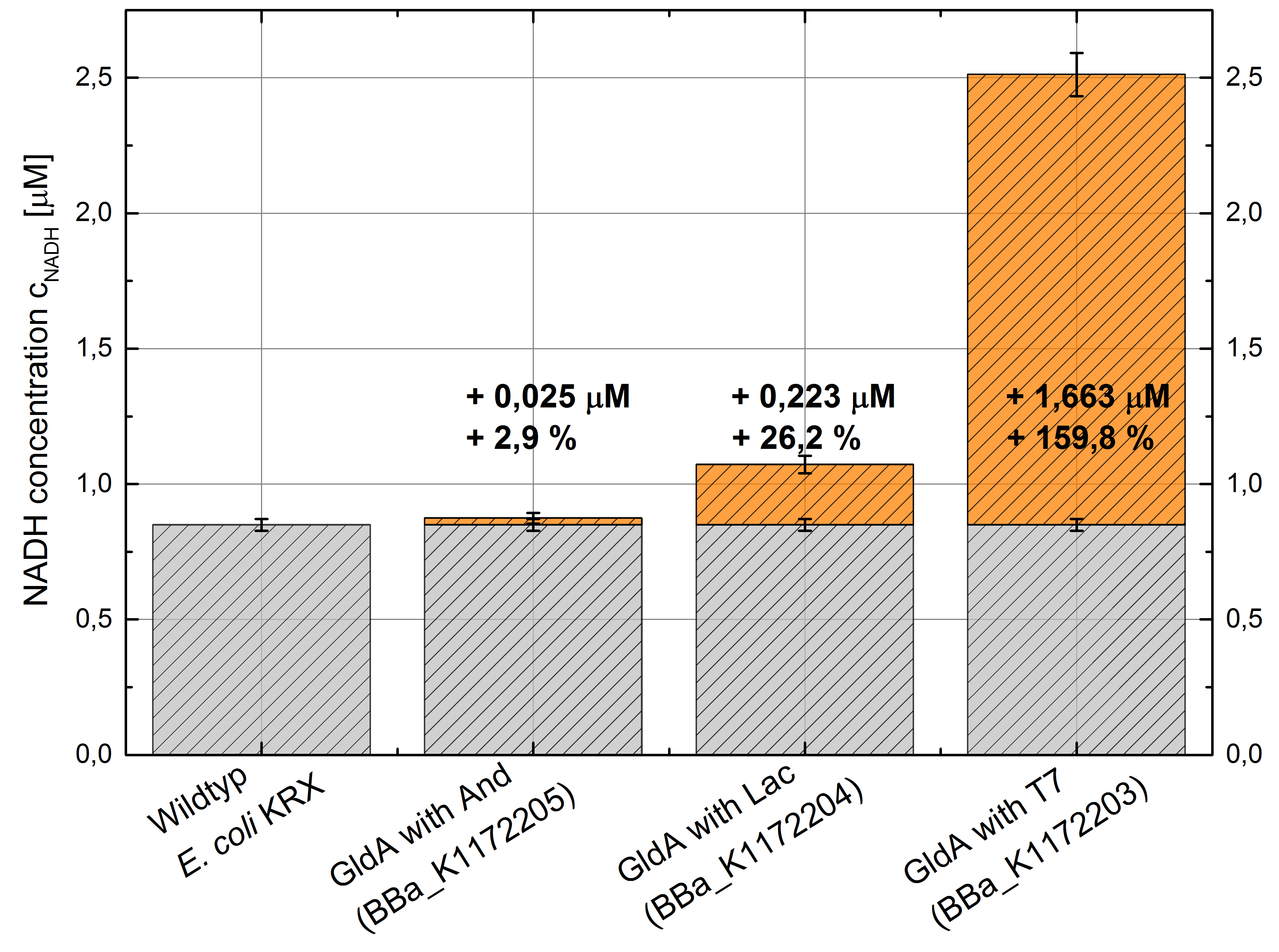
Figure 5: Column Chart with results of the NADH-assay. Comparison between Escherichia coli KRX wild typ and Escherichia coli KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart>. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.
Compared with Escherichia coli KRX wild type, Escherichia coli with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) shows 1,7 μM NADH overproduction and GldA with Lac promoter (<bbpart>BBa_K1172204</bbpart>) shows 0,2 μM NADH overproduction. The GldA expression by the Anderson promoter (<bbpart>BBa_K1172205</bbpart>) is too weak for an efficient NADH overproduction.
Thus, E. coli with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) is used for all further tests, because this strain shows by far the best NADH production rate.
Former MFC tests showed, that glycerol is the best carbon source for Escherichia coli in our Microbial Fuel Cell. Therefore it is important to see, which effect an increased glycerol concentration on the mediator production has. To test these parameters, LB medium was supplemented with different amounts of glycerol. (Table 3)
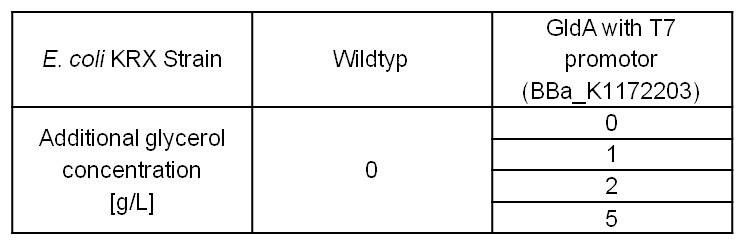
Table 3: Experimental design of the glycerol dependent NADH-assay. Supplementation of different amounts of glycerol to LB medium should show the effect of glycerol on NADH overproduction.
E.coli grown in glycerol supplemented medium shows great enhanced NADH production in contrast to Escherichia coli KRX wild type and also in contrast to Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> and no further NADH supplementation. (Table 4 and Figure 6)
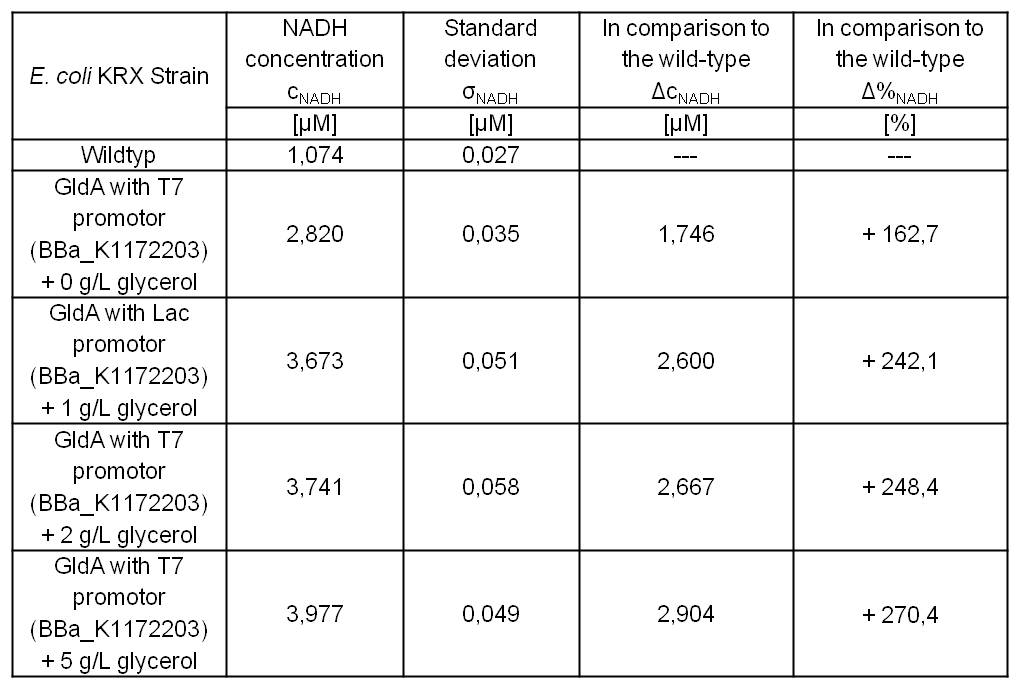
Table 4: Results of the NADH-assay. Comparison between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> supplemented with different amounts of glycerol to LB medium. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.
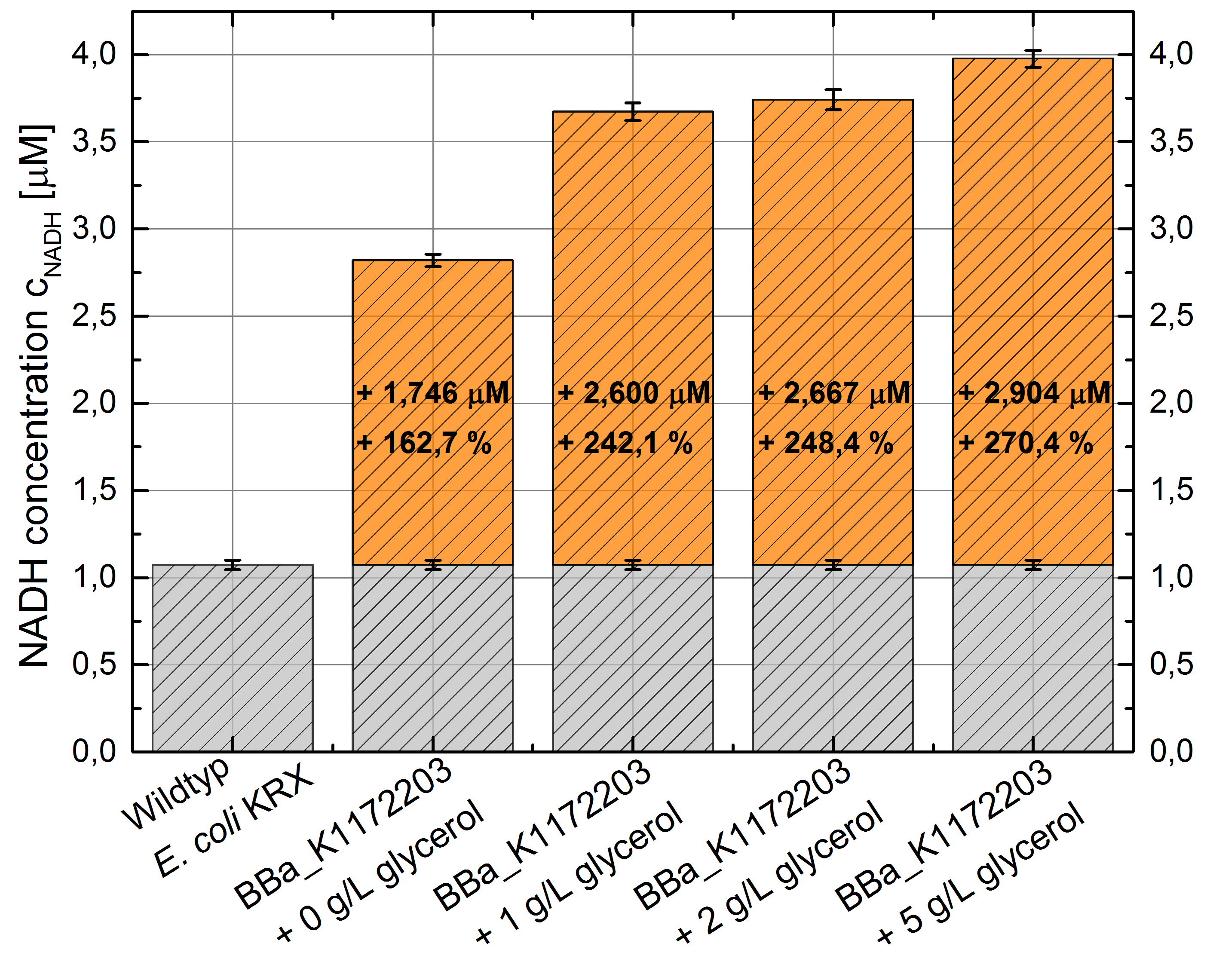
Figure 6: Column Chart with results of the NADH-assay. Comparison between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> supplemented with different amounts of glycerol to LB medium. NADH concentration with standard deviation and concentration in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.
Glycerol dependent NADH-assay shows a quiet good NADH overproduction for <bbpart>BBa_K1172203</bbpart> with supplementation of glycerol. It was possible to increase NADH production up to 270 % in comparison to Escherichia coli KRX wild type and up to 100 % in comparison to Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> and without glycerol supplementation. However, it is relatively unimportant how much glycerol is supplemented to the medium. However, this growth characteristics is initially only valid for LB medium glycerol supplementation.
With former NADH-assays we only observed the effects of GldA on the intracellular NADH concentration of E. coli. To investigate additionally extracellular NADH concentration, we tested Escherichia coli KRX wild type strain and E. coli KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart> by cultivation with different glycerol concentrations in M9 medium. (Table 5)
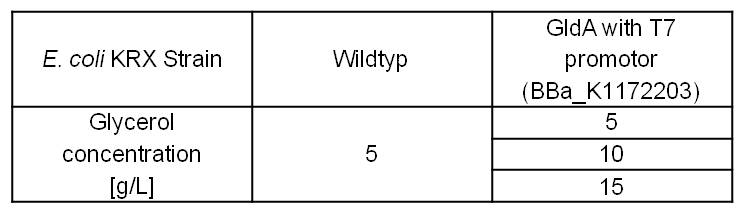
Table 5: Experimental design of the glycerol dependent NADH-assay. Different concentrations of glycerol in M9 medium should show the effect of glycerol on NADH overproduction.
Cultivating Escherichia coli <bbpart>BBa_K1172203</bbpart> with increasing glycerol concentration of M9 medium shows enhanced intracellular and also enhanced extracellular NADH concentration in contrast to Escherichia coli KRX wild type. (Table 6 and Figure 7)
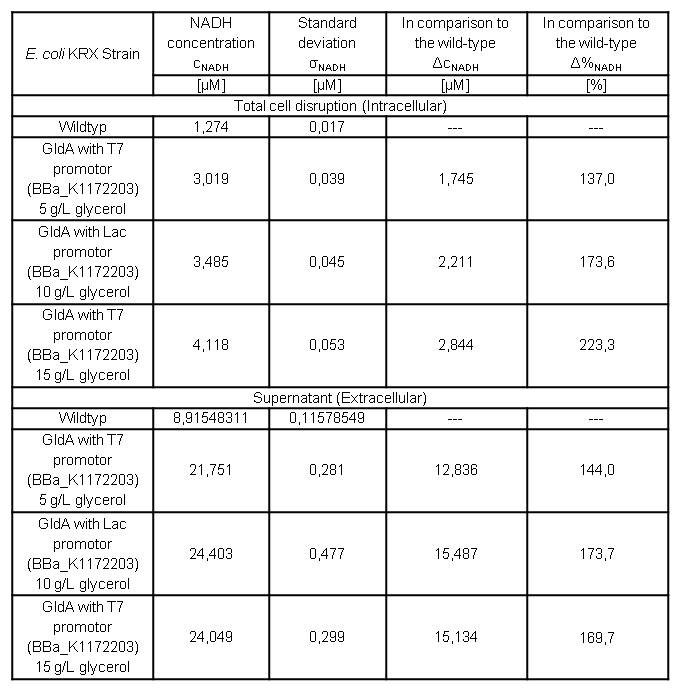
Table 6: Results of the NADH-assay. Comparison between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> grown in M9 medium with different concentrations of glycerol. NADH concentration with standard deviation and concentration in comparison to the wild type is shown for cell disruption and supernatant fraction. Two biological and at least three technical replicates were analyzed.
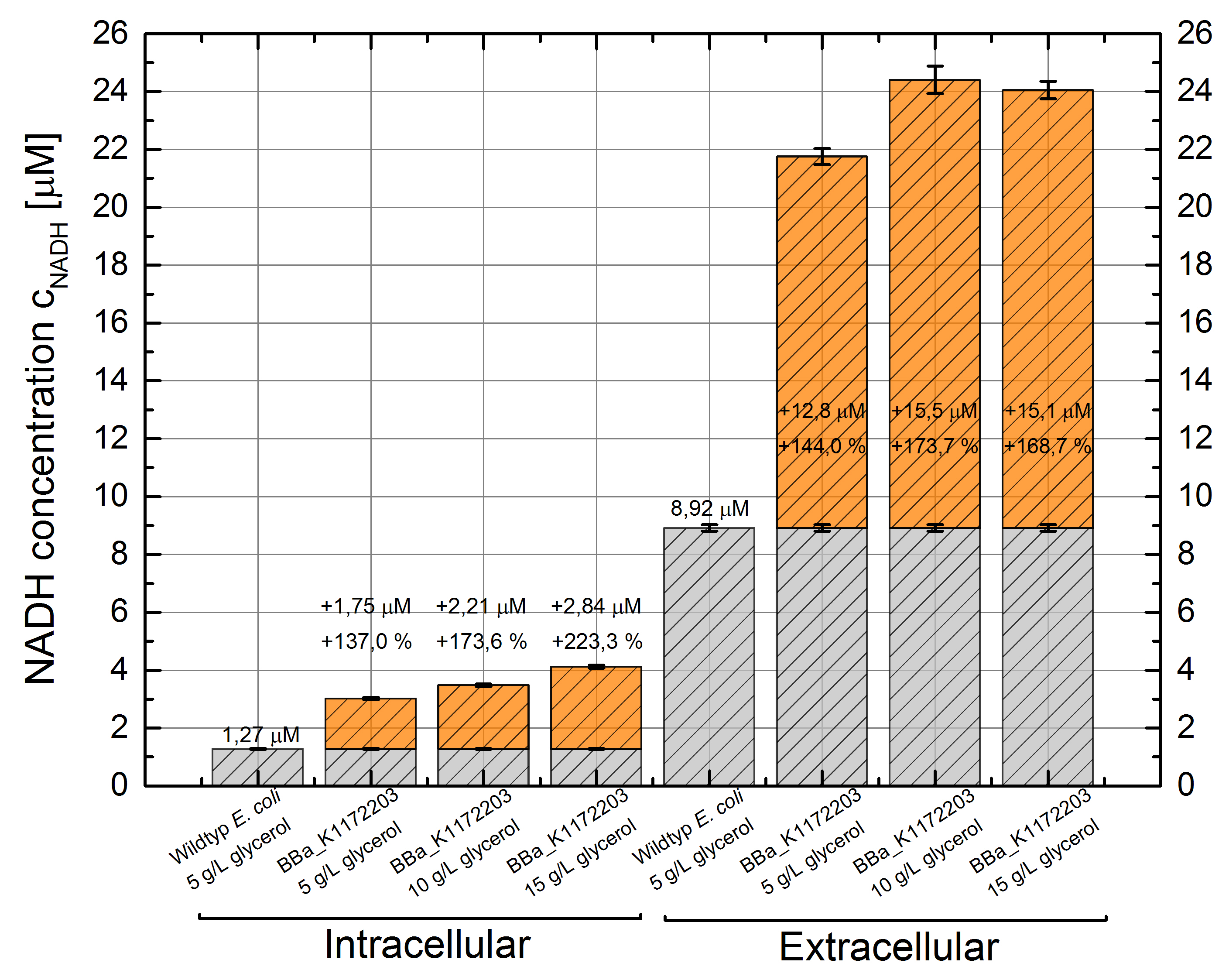
Figure 7: Column Chart with results of the NADH-assay. Comparison between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> grown in M9 medium with different concentrations of glycerol. NADH concentration with standard deviation and concentration in comparison to the wild type is shown for cell disruption (Intracellular) and supernatant (Extracellular) fraction. Two biological and at least three technical replicates were analyzed.
In addition to previously observed increase in intracellular NADH concentration, we were able to show an enhanced extracellular NADH concentration. Heterologous expression of GldA <bbpart>BBa_K1172203</bbpart> in E. coli seems to be an appropriate way for endogenous mediator production. Furthermore glycerol could be confirmed as a suitable substrate for the NADH production.
Intracellular NADH concentration increases up to 223 % in comparison to Escherichia coli KRX wild type with increasing promoter strength. Besides it is very interesting to see that extracellular NADH concentration is 7 times higher than the intracellular concentration for all strains. Heterologous expression of GldA <bbpart>BBa_K1172203</bbpart> in E. coli causes a 2.5 times higher extracellular NADH concentration compared with Escherichia coli KRX wild type.
These data show that NADH is an adequate mediator for Microbial Fuel Cells. NADH can be transported across the cell membrane which is indicated by much higher extracellular NADH concentration and thus allow NADH-mediated electron transfer (EET).
Microbial Fuel Cell Measurement
All NADH-assays showed great enhanced NADH overproduction for GldA strain. Therefore higher electron transport efficiency should result in an improved bioelectricity output.
For testing the genetic engineered system in the Microbial Fuel Cell, we used Escherichia coli KRX with GldA and Lac promoter (<bbpart>BBa_K1172204</bbpart>) in contrast to Escherichia coli KRX wild type. Certainly NADH-assays determined Escherichia coli KRX with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) as the best endogenous mediator producing strain. Unfortunately we could not use this strain due to cultivation problems.
According to our assumptions, the extracellular electron transfer mediated by NADH is improved in the GldA strain resulting in an increased bioelectricity output. (Figure 8 and 9)
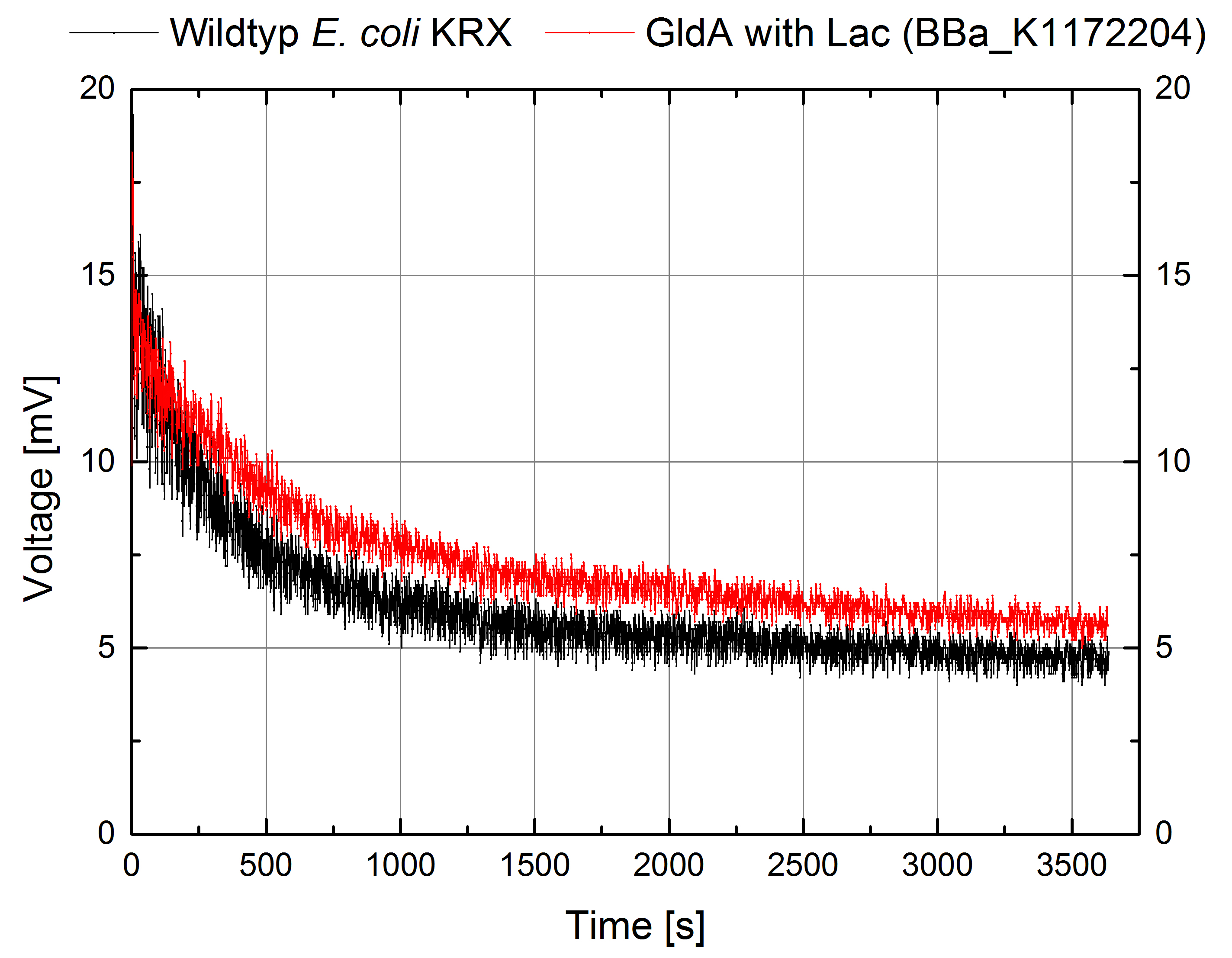
Figure 8: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with GldA (<bbpart>BBa_K1172204</bbpart>) in contrast to Escherichia coli KRX wild type. Voltage curve from Escherichia coli KRX wild type and Escherichia coli KRX with GldA (<bbpart>BBa_K1172204</bbpart>) is shown over time over a resistance of 200 Ω. M9-medium was used with no supplementation of mediators.
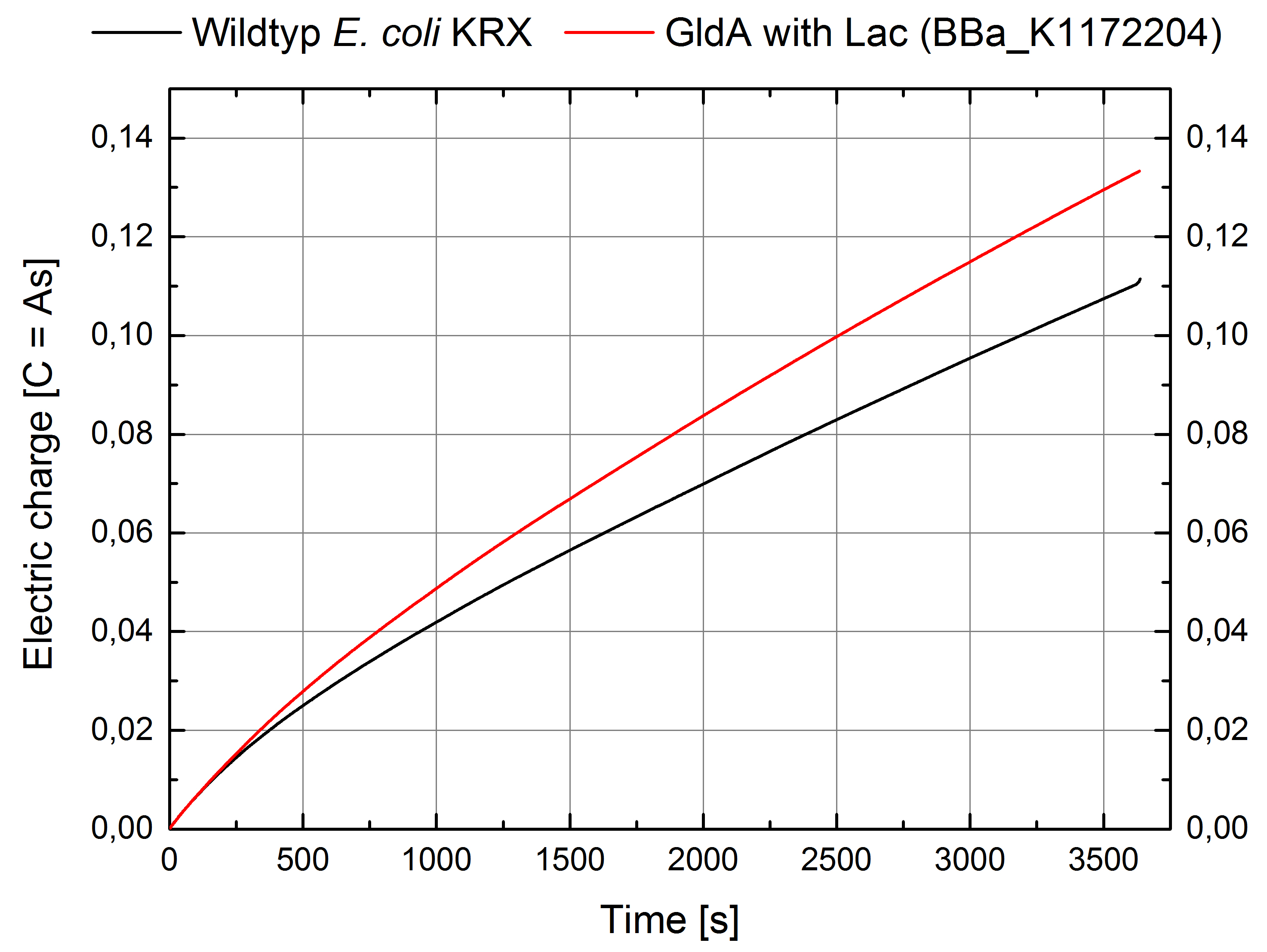
Figure 9: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with GldA (<bbpart>BBa_K1172204</bbpart>) in contrast to Escherichia coli KRX wild type. Electric charge curve from Escherichia coli KRX wild type and Escherichia coli KRX with GldA (<bbpart>BBa_K1172204</bbpart>) is shown over time over a resistance of 200 Ω. M9-medium was used with no supplementation of mediators.
Escherichia coli KRX with GldA (<bbpart>BBa_K1172204</bbpart>) shows 27 % higher maximal voltage than Escherichia coli KRX wild type. Over the whole cultivation, voltage was about 20% improved. The calculation of the electric charge confirms the described results. Electric charge is equivalent to the number of transported electrons and 20 % enhanced for Escherichia coli KRX with GldA (<bbpart>BBa_K1172204</bbpart>). The maximal electric charge of 135 mC examines that overexpression of glycerol dehydrogenase enables endogenous mediator production and more efficient electron shuttle-mediated electron transfer.
A further optimization by using Escherichia coli KRX with GldA and T7 promoter (<bbpart>BBa_K1172203</bbpart>) instead of Lac promoter (<bbpart>BBa_K1172203</bbpart>) would improve electricity output according NADH-assay results (Table 2 and Figure 5) of about 134 %. These results demonstrate that the redox mediator NADH from Escherichia coli facilitates the electron transfer between cell and electrode and shows a starting point for reducing costs by expensive and toxix exogenous mediators.
Conclusion
Mediators are essential for the use of Escherichia coli in Microbial Fuel Cells. The main advantage of improving MFCs is to enhance kinetics of the electron transfer between the bacterial cells and the fuel cell anode. In order to decrease the usage of expensive and toxic synthetic mediators (exogenous mediators like methylene blue oder neutral red), we produced the endogenous mediator NADH by overexpression of glycerol dehydrogenase.
Looking at our project in its entirety, the overproduction of glycerol dehydrogenase seems to be a great way for endogenous mediator production. The use of glycerol as our main carbon source for Escherichia coli in the MFC further enhances the efficiency of NADH production. We can show a 270 % higher intracellular NADH concentration (4 μM) for Escherichia coli KRX with GldA plasmid and 170 % higher extracellular NADH concentration (24 μM) in contrast to Escherichia coli KRX wild type.
The heterologous expression of GldA is a great genetic strategy to optimize mediator production as well as electricity generation in Microbial Fuel Cells. The most suitable and efficient GldA device for Escherichia coli is a combination with Rhamnose inducible T7 promoter (<bbpart>BBa_K1172203</bbpart>).
References
Kelley JJ, Dekker EE (1985) Identity of Escherichia coli D-1-amino-2-propanol: NAD+ oxidoreductase with E. coli glycerol dehydrogenase but not with Neisseria gonorrhoeae 1,2-propanediol:NAD+ oxidoreductase. [http://jb.asm.org/content/162/1/170.short| J. Bacteriol. 162: 170].
Mulichak AM (2005) Crystal structure of glycerol dehydrogenase. [http://www.rcsb.org/pdb/images/1ta9_bio_r_500.jpg| Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Band (PDB)].
Park DH, Kim SK, Shin IH, Jeong YJ (2000) Electricity production in biofuel cell using modified graphite electrode with Neutral Red. [http://link.springer.com/article/10.1023/A:1005674107841#page-1| Biotechnol. Lett 22: 1301].
Qiao Y, Li CM, Bao SJ, Lu ZS, Hong YH (2008) Electrocatalysis in microbial fuel cells - from electrode material to direct electrochemistry. [http://pubs.rsc.org/en/content/articlehtml/2010/ee/b923503e| Chem. Commun. 1290].
Ruzheinikov SN, Burke J, Sedelnikova S, Baker PJ, Taylor B, Bullough PA, Muir NM, Gore MG, Rice DW (2001) Glycerol Dehydrogenase: Structure, Specificity, and Mechanism of a Family III Polyol Dehydrogenase. [http://www.sciencedirect.com/science/article/pii/S0969212601006451|Structure 9: 789–802].
Seop CI, Moon H, Bretschger O, Jang JK, Park HI, Nealson KH, Kim BH (2006) Electrochemically Active Bacteria (EAB) and Mediator-Less Microbial Fuel Cells. [http://www.microbialfuelcell.org/Publications/EBL/1-10/8_2006_Chang%20I.%20S_%20Electrochemically%20active%20bacteria%20%28EAB%29%20and%20mediator-less%20microbial%20fuel%20cells.pdf|J. Microbiol. Biotechnol. 16 (2): 163–177].
Xiang K, Qiao Y, Ching CB, Li CM (2009) GldA overexpressing-engineered E. coli as superior electrocatalyst for microbial fuel cells. [http://www.sciencedirect.com/science/article/pii/S1388248109002835| Electrochemistry Communications 11: 1593–1595].
Zhang T, Cui C, Chen S, Ai X, Yang H, Shen P, Peng Z (2006) A novel mediatorless microbial fuel cell based on direct biocatalysis of Escherichia coli. [http://pubs.rsc.org/en/content/articlehtml/2006/cc/b600876c| Chem Commun 11: 2257–2259].
 "
"