Team:Paris Saclay/Notebook/August/2
From 2013.igem.org
(Difference between revisions)
(→Objective : obtaining BBa_K1155007) |
(→Transformation the Gibson assembly mix (August 1st)) |
||
| (12 intermediate revisions not shown) | |||
| Line 73: | Line 73: | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL of DNA Ladder | * Well 1 : 6µL of DNA Ladder | ||
| - | * Well 2 et 3 : 5µL of BBa_I732017 digested by EcoRI/SpeI+1µL 6X loading dye | + | * Well 2 et 3 : 5µL of BBa_I732017 digested by EcoRI/SpeI + 1µL 6X loading dye |
* Gel : 0.8% | * Gel : 0.8% | ||
|} | |} | ||
| Line 79: | Line 79: | ||
Expected sizes : | Expected sizes : | ||
* RBS-LacZ : 3093 bp | * RBS-LacZ : 3093 bp | ||
| - | * | + | * pSB1A2 : 2079 bp |
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We didn't obtain fragments at the right size. We will digest BBa_KI732017 again. |
|} | |} | ||
| Line 89: | Line 89: | ||
===='''Objective : obtaining FNR and BphR2 proteins'''==== | ===='''Objective : obtaining FNR and BphR2 proteins'''==== | ||
| + | XiaoJing | ||
| - | ====''' | + | ====''' Transformation the Gibson assembly mix (August 1st)'''==== |
| - | + | * plasmid pSB1C3 containing BphR2 | |
| + | * plasmid pSB1C3 containing FNR | ||
| + | * plasmid pSB1C3 contaning RBS-FNR in competent cell DH5α | ||
Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | ||
Latest revision as of 21:15, 4 October 2013
Notebook : August 2
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155003
1 - Extraction of BBa_K1155003 from DH5α
Damir, Nadia
|
Tranformation from 07/30/13 works. We will extract plasmid BBa_K1155003. |
Protocol : High-copy plasmid extraction
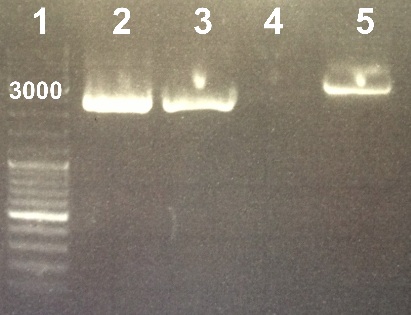
We extracted plasmid from colony 9, 11 and 12. We eluted our extracted plasmid in 50µL H2O.
2 - Electrophoresis to check the extraction of BBa_K1155003
Damir, Nadia
Expected sizes :
- BBa_K1155003 : 2734 pb
Estimated concentrations :
- Clone 9 : 18ng/µL
- Clone 11 :18ng/µL
- Clone 12 : 18ng/µL
|
We obtained fragments at the right size (Clone 12 starts 5 min after the others). The extraction was good. We will digest it. |
Objective : obtaining BBa_K1155007
1 - Digestion of BBa_I732017 by EcoRI/SpeI to check sizes of fragments
Damir, Nadia
Used quantities :
- BBa_I732017 : 41µL
- Buffer FD : 5µL
- EcoRI : 2µL
- SpeI : 2µL
We let the digestion at 1h30 at 37°C.
2 -Electrophoresis to check the digestion of BBa_I732017 by EcoRI/SpeI
Damir, Nadia
|
|
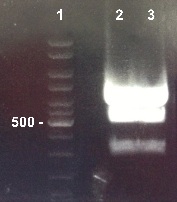
Expected sizes :
- RBS-LacZ : 3093 bp
- pSB1A2 : 2079 bp
|
We didn't obtain fragments at the right size. We will digest BBa_KI732017 again. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
XiaoJing
Transformation the Gibson assembly mix (August 1st)
- plasmid pSB1C3 containing BphR2
- plasmid pSB1C3 containing FNR
- plasmid pSB1C3 contaning RBS-FNR in competent cell DH5α
Protocol : Bacterial transformation
| Previous day | Back to calendar | Next day |
 "
"