Team:Paris Saclay/Notebook/August/5
From 2013.igem.org
(→3 - PCR of PSB1C3) |
(→3 - Electrophoresis of PCR of pSB1C3) |
||
| (10 intermediate revisions not shown) | |||
| Line 31: | Line 31: | ||
* Well 1 : 6µL of DNA Ladder | * Well 1 : 6µL of DNA Ladder | ||
* Well 2 : 30µL of BBa_K1155003 from clone 9 digested by EcoRI/PstI + 6µl of 6X loading dye | * Well 2 : 30µL of BBa_K1155003 from clone 9 digested by EcoRI/PstI + 6µl of 6X loading dye | ||
| - | * Well 3 : 5µL of BBa_K1155003 from clone 9 +1µl of 6X loading dye | + | * Well 3 : 5µL of BBa_K1155003 from clone 9 + 1µl of 6X loading dye |
* Well 4 : 30µL of BBa_K1155003 from clone 11 digested by EcoRI/PstI + 6µl of 6X loading dye | * Well 4 : 30µL of BBa_K1155003 from clone 11 digested by EcoRI/PstI + 6µl of 6X loading dye | ||
| - | * Well 5 : 5µL of BBa_K1155003 from clone 11 +1µl of 6X loading dye | + | * Well 5 : 5µL of BBa_K1155003 from clone 11 + 1µl of 6X loading dye |
* Well 6 : 30µL of BBa_K1155003 from clone 12 digested by EcoRI/PstI +6µl of 6X loading dye | * Well 6 : 30µL of BBa_K1155003 from clone 12 digested by EcoRI/PstI +6µl of 6X loading dye | ||
| - | * Well 7 : 5µL of BBa_K1155003 from clone 12 +1µl of 6X loading dye | + | * Well 7 : 5µL of BBa_K1155003 from clone 12 + 1µl of 6X loading dye |
* Gel : 1% | * Gel : 1% | ||
|} | |} | ||
| Line 45: | Line 45: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We obtained fragments at the right size. We will sequence it. |
|} | |} | ||
| Line 80: | Line 80: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We obtained fragments at the right size. We will make an electro-elution to extract RBS-LacZ. |
|} | |} | ||
| Line 100: | Line 100: | ||
Hybridation temperature gradient : | Hybridation temperature gradient : | ||
| - | A - | + | A - 65°C/ |
| - | B - 64. | + | B - 64.7°C/ |
| - | C - 64. | + | C - 64.1°C/ |
| - | D - 63. | + | D - 63.1°C/ |
| - | E - | + | E - 62°C/ |
| - | F - 61. | + | F - 61.2°C/ |
| - | G - 60. | + | G - 60.5°C/ |
| - | H - | + | H - 60°C/ |
[[File:PsPCRC30508.jpg|400px]] | [[File:PsPCRC30508.jpg|400px]] | ||
| Line 119: | Line 119: | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL of DNA Ladder | * Well 1 : 6µL of DNA Ladder | ||
| - | * Well 2 to 9 : 2µL of pSB1C3+3µL of H2O+1µL of 6X loading dye | + | * Well 2 to 9 : 2µL of pSB1C3 + 3µL of H2O + 1µL of 6X loading dye |
* Gel : 1% | * Gel : 1% | ||
|} | |} | ||
| Line 128: | Line 128: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We obtained fragments at the right size. We will purify it. |
|} | |} | ||
| Line 139: | Line 139: | ||
===='''Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== | ===='''Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== | ||
| - | ===='''1 - | + | ===='''1 - PCR Colonies of BBa_K1155004, BBa_K115005, BBa_K1155006 in DH5α'''==== |
Damir, Nadia, XiaoJing | Damir, Nadia, XiaoJing | ||
| Line 148: | Line 148: | ||
|} | |} | ||
| - | We | + | We took a single colony and resuspend in 10µL H2O. For each Biobrick, we did 25 PCR Colonies. |
Used quantities : | Used quantities : | ||
| - | + | ||
| - | + | PCR preparation mix for 25 different colonies including: | |
** Oligo 44 : 3.5µL | ** Oligo 44 : 3.5µL | ||
** Oligo 45 : 3.5µL | ** Oligo 45 : 3.5µL | ||
| Line 159: | Line 159: | ||
** Dream Taq : 5µL | ** Dream Taq : 5µL | ||
** H2O : 590µL | ** H2O : 590µL | ||
| + | |||
| + | PCR reaction: | ||
| + | * DNA : 2µL of resuspend colony | ||
| + | * Mix PCR :23µL | ||
| + | Total volume: 25µL | ||
PCR Program : | PCR Program : | ||
Latest revision as of 01:25, 5 October 2013
Notebook : August 5
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155003
1 - Digestion of BBa_K1155003 by EcoRI/PstI to check the ligation between RBS-Amil CP,Term and pSB1C3
Nadia, XiaoJing
Used quantities :
- BBa_K1155003 : 5µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- Buffer FD : 3µL
- H2O : 20µL
We incubate the digestion at 37°C during 15 minutes.
2 - Electrophoresis of the digestion of BBa_K1155003
Damir
Expected sizes :
- RBS_AmilCP-Term : 824bp
- pSB1C3 : 2070bp
|
We obtained fragments at the right size. We will sequence it. |
Objective : obtaining BBa_K1155007
1 - Digestion of BBa_I732017 by EcoRI/SpeI
Abdou, Damir, Nadia,
Used quantities :
- BBa_I732017 : 41µL
- Buffer FD : 5µL
- EcoRI : 2µL
- SpeI : 2µL
We incubate the digestion at 37°C for 1h30.
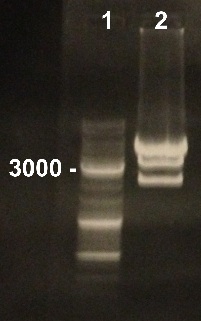
2 -Electrophoresis of the digestion of BBa_I732017 by EcoRI/SpeI
Damir, Nadia
|
|
Expected sizes :
- RBS-LacZ : 3093 bp
- pSB1A2 : 2079 bp
|
We obtained fragments at the right size. We will make an electro-elution to extract RBS-LacZ. |
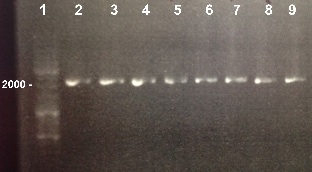
3 - PCR of pSB1C3
Nadia, XiaoJing
Used quantities :
- pSB1C3 : 2µL
- Buffer phusion : 10µL
- Oligo 64 : 2.5µL
- Oligo 65 : 2.5µL
- dNTP : 1µL
- enzyme Phusion : 0.25µL
- H2O : 36.75µL
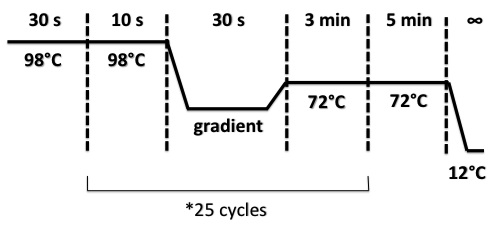
PCR program :
Hybridation temperature gradient :
A - 65°C/ B - 64.7°C/ C - 64.1°C/ D - 63.1°C/ E - 62°C/ F - 61.2°C/ G - 60.5°C/ H - 60°C/
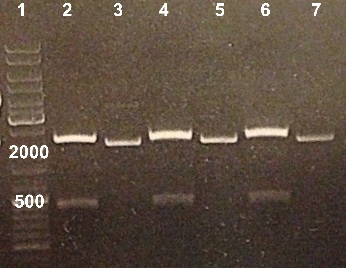
3 - Electrophoresis of PCR of pSB1C3
Nadia, XiaoJing
|
|
Expected sizes :
- pSB1C3 : 2070bp
|
We obtained fragments at the right size. We will purify it. |
4 - Gel purification of electrophoresis of PCR of pSB1C3
Nadia, XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - PCR Colonies of BBa_K1155004, BBa_K115005, BBa_K1155006 in DH5α
Damir, Nadia, XiaoJing
|
Transformation of 07/31/13 works. We will do a PCR Colony. |
We took a single colony and resuspend in 10µL H2O. For each Biobrick, we did 25 PCR Colonies.
Used quantities :
PCR preparation mix for 25 different colonies including:
- Oligo 44 : 3.5µL
- Oligo 45 : 3.5µL
- Buffer Dream Taq : 70µL
- dNTP : 28µL
- Dream Taq : 5µL
- H2O : 590µL
PCR reaction:
- DNA : 2µL of resuspend colony
- Mix PCR :23µL
Total volume: 25µL
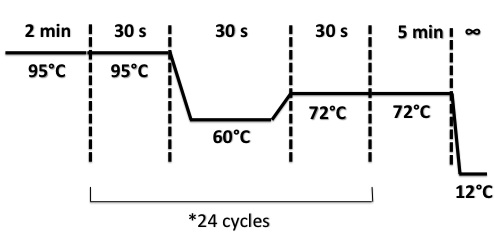
PCR Program :
| Previous day | Back to calendar | Next day |
 "
"