Team:Paris Saclay/Notebook/August/6
From 2013.igem.org
(Difference between revisions)
(→1 - Electrophoresis to check the PCR Colonies products : BBa_K1155004, BBa_K1155005, BBa_K1155006) |
|||
| (29 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
==='''A - Aerobic/Anaerobic regulation system'''=== | ==='''A - Aerobic/Anaerobic regulation system'''=== | ||
| - | ====''' | + | ===='''Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== |
| + | |||
| + | ====1 - Electrophoresis to check the PCR Colonies products : BBa_K1155004, BBa_K1155005, BBa_K1155006==== | ||
| - | |||
XiaoJing, Damir, Anaïs | XiaoJing, Damir, Anaïs | ||
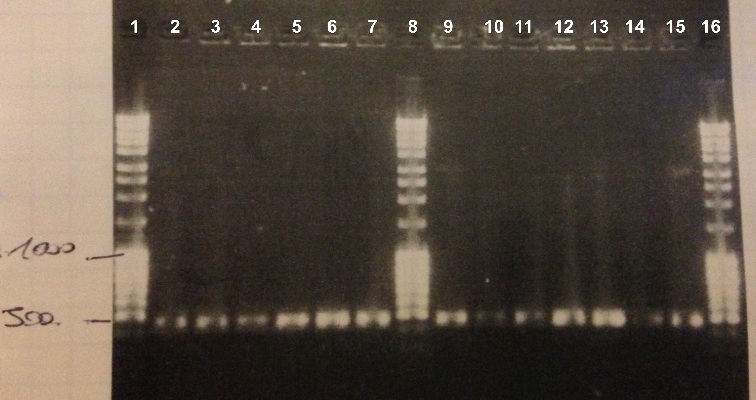
| - | * | + | * BBa_K1155004 : |
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" | | + | | style="width:350px;border:1px solid black;" |[[File:Psgel10608.jpg| 400px]][[File:Psgel20608.jpg| 400px]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| - | * | + | * Well 1 : 6µL DNA Ladder |
| - | *Well | + | * Well 2 to 7 : 10µL BBa_K1155004 + 2µl of 6X loading dye |
| - | * | + | * Well 8 : 6µL DNA Ladder |
| - | + | * Well 9 to 15 : 10µL BBa_K1155004 + 2µl of 6X loading dye | |
| + | * Well 16 : 6µL DNA Ladder | ||
| - | |||
| - | + | * Well 1 : 6µL DNA Ladder | |
| + | * Well 2 to 7 : 10µL BBa_K1155004 + 2µl of 6X loading dye | ||
| + | * Well 8 : 6µL DNA Ladder | ||
| + | * Well 9 to 14 : 10µL BBa_K1155004 + 2µl of 6X loading dye | ||
| + | * Well 15 : 6µL DNA Ladder | ||
| + | * Gel : 1% | ||
| + | |} | ||
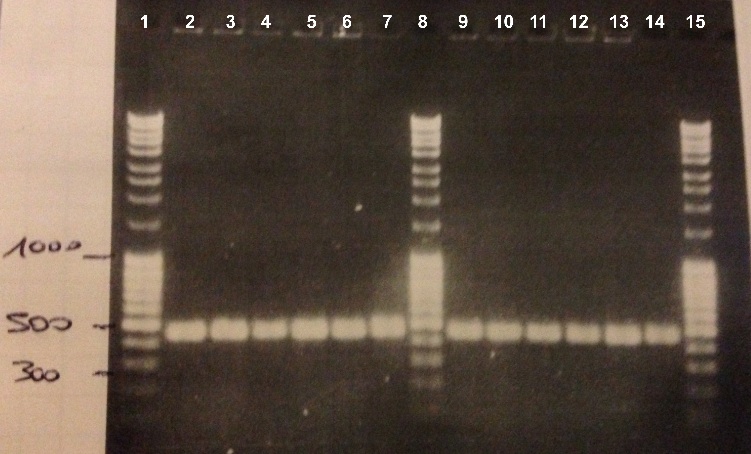
| - | * | + | *BBa_K1155005 : |
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" | | + | | style="width:350px;border:1px solid black;" |[[File:Psgel30608.jpg| 400px]][[File:Psgel40608.jpg| 400px]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| - | * | + | * Well 1 : 6µL DNA Ladder |
| - | * | + | * Well 2 to 7 : 10µL BBa_K1155005 + 2µl of 6X loading dye |
| - | + | * Well 8 : 6µL DNA Ladder | |
| - | * | + | * Well 9 to 14 : 10µL BBa_K1155005 + 2µl of 6X loading dye |
| - | * | + | * Well 15 : 6µL DNA Ladder |
| - | + | ||
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
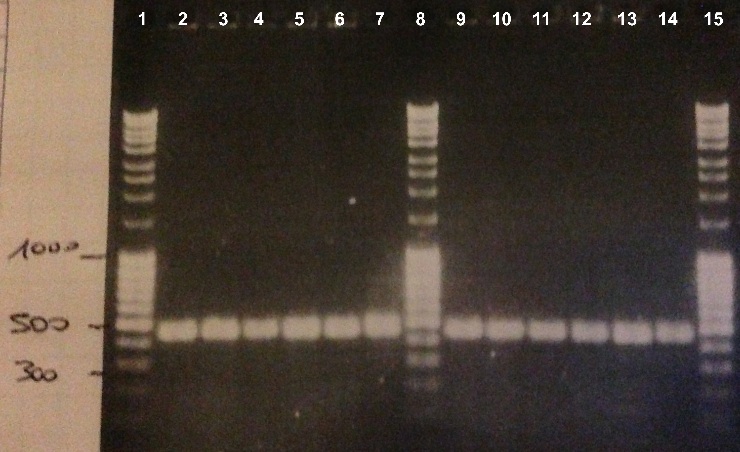
| - | + | * Well 1 : 6µL DNA Ladder | |
| + | * Well 2 to 7 : 10µL BBa_K1155005 + 2µl of 6X loading dye | ||
| + | * Well 8 : 6µL DNA Ladder | ||
| + | * Well 9 to 15 : 10µL BBa_K1155005 + 2µl of 6X loading dye | ||
| + | * Well 16 : 6µL DNA Ladder | ||
| + | * Gel : 1% | ||
| + | |} | ||
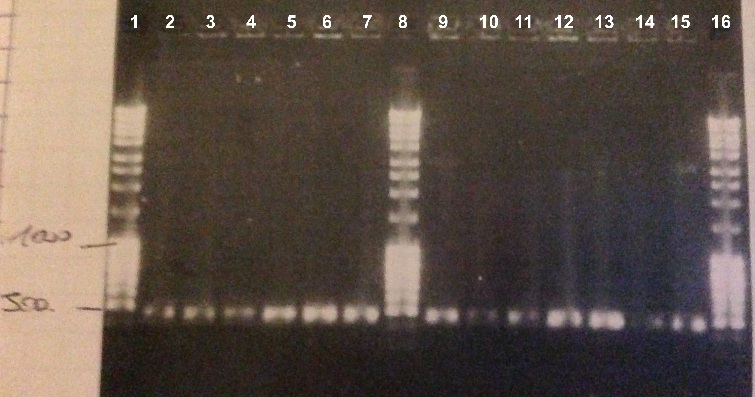
| - | * | + | *BBa_K1155006 : |
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" | | + | | style="width:350px;border:1px solid black;" |[[File:Psgel50608.jpg| 400px]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
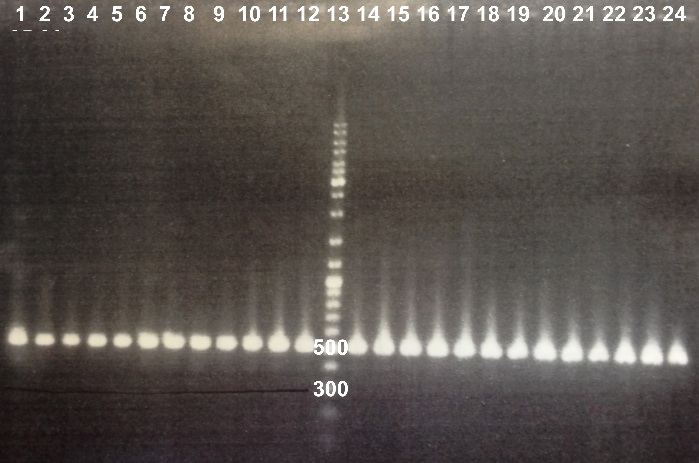
| - | * | + | * Well 1 to 12 : 10µL BBa_K1155006+2µl of 6X loading dye |
| - | + | * Well 13 : 6µL DNA Ladder | |
| - | + | * Well 14 to 26 : 10µL BBa_K1155006+2µl of 6X loading dye | |
| - | + | * Gel : 1% | |
| - | * | + | |
| - | + | ||
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | *Gel : 1 | + | |
|} | |} | ||
| - | Expected size : 500 bp | + | Expected size : |
| + | * NarK, NarG, NirB : 500 bp | ||
| - | + | {| | |
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtained fragments of the good size for all the colonies. We will make a culture for DH5α strain containing BBa_K1155004, BBa_K1155005 and BBa_K1155006. We will also sequence our plasmids. | ||
| + | |} | ||
| + | |||
| + | ====2 - Liquid culture of DH5αstrain containing BBa_K1155004, BBa_K1155005 and BBa_K1155006 ==== | ||
| - | |||
Xiaoing, Anaïs | Xiaoing, Anaïs | ||
| - | + | Used quantities : | |
| - | + | * LB : 5mL | |
| + | * Chloramphenicol (1000X, 20µg/mL) : 5µL | ||
| + | * Strain containing BBa_K1155004, BBa_K1155005 and BBa_K1155006 : 25µL | ||
| + | |||
| + | We incubate the cultures over night at 37°C with agigation at 180 RPM. | ||
| + | |||
| + | We picked colonies number 6, 7 and 8 for each promoter. | ||
| + | |||
| + | ===='''Objective : obtaining BBa_K1155007'''==== | ||
| + | |||
| + | ====1 - Electroelution of BBa_I732017 digested by EcoRI/SpeI==== | ||
| + | |||
| + | Nadia | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/electro|Electroelution]] | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | The electroelution was good. We will ligate RBS-LacZ with Term and pSB1C3. | ||
| + | |} | ||
| + | |||
| + | ===='''Objective : obtaining biobricks in pSB3K3'''==== | ||
| + | |||
| + | ====1 - Extraction of BBa_J04450 from DH5α==== | ||
| + | |||
| + | Abdou | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/extraction|Low copy plamid extraction]] | ||
==='''B - PCB sensing system'''=== | ==='''B - PCB sensing system'''=== | ||
| - | ====''' | + | ===='''Objective : obtaining BBa_K1155002'''==== |
| - | ====1 - | + | ====1 - Sequence analysis for BBa_K1155002 in clones 4, 17 and 22==== |
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | The sequence is good for each clone. We obtain a new biobrick : BBa_K1155002. | ||
| + | |} | ||
| + | |||
| + | {| border="1" align="center" | ||
| + | |[[Team:Paris Saclay/Notebook/August/5|<big>Previous day</big>]] | ||
| + | |||
| + | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| + | |||
| + | |[[Team:Paris Saclay/Notebook/August/7|<big>Next day</big>]] | ||
| + | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 01:25, 5 October 2013
Notebook : August 6
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - Electrophoresis to check the PCR Colonies products : BBa_K1155004, BBa_K1155005, BBa_K1155006
XiaoJing, Damir, Anaïs
- BBa_K1155004 :
- BBa_K1155005 :
- BBa_K1155006 :
|
|
Expected size :
- NarK, NarG, NirB : 500 bp
|
We obtained fragments of the good size for all the colonies. We will make a culture for DH5α strain containing BBa_K1155004, BBa_K1155005 and BBa_K1155006. We will also sequence our plasmids. |
2 - Liquid culture of DH5αstrain containing BBa_K1155004, BBa_K1155005 and BBa_K1155006
Xiaoing, Anaïs
Used quantities :
- LB : 5mL
- Chloramphenicol (1000X, 20µg/mL) : 5µL
- Strain containing BBa_K1155004, BBa_K1155005 and BBa_K1155006 : 25µL
We incubate the cultures over night at 37°C with agigation at 180 RPM.
We picked colonies number 6, 7 and 8 for each promoter.
Objective : obtaining BBa_K1155007
1 - Electroelution of BBa_I732017 digested by EcoRI/SpeI
Nadia
Protocol : Electroelution
|
The electroelution was good. We will ligate RBS-LacZ with Term and pSB1C3. |
Objective : obtaining biobricks in pSB3K3
1 - Extraction of BBa_J04450 from DH5α
Abdou
Protocol : Low copy plamid extraction
B - PCB sensing system
Objective : obtaining BBa_K1155002
1 - Sequence analysis for BBa_K1155002 in clones 4, 17 and 22
|
The sequence is good for each clone. We obtain a new biobrick : BBa_K1155002. |
| Previous day | Back to calendar | Next day |
 "
"