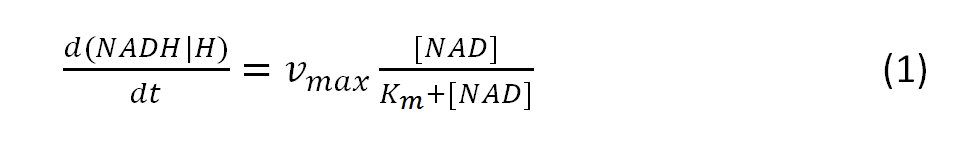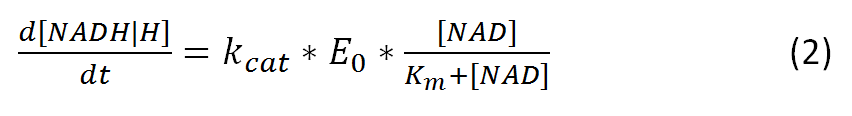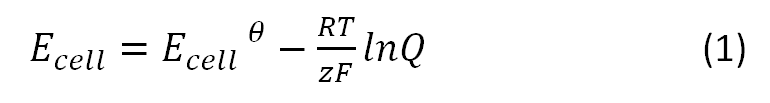Team:Bielefeld-Germany/Modeling
From 2013.igem.org
| (13 intermediate revisions not shown) | |||
| Line 81: | Line 81: | ||
==Intermediates== | ==Intermediates== | ||
<p align="justify"> | <p align="justify"> | ||
| - | The electrons that will be eventually transferred to the anode are generated during the biochemical processes within the bacterial cell. The central pathway in E.coli is the glycolysis and one of its | + | The electrons that will be eventually transferred to the anode are generated during the biochemical processes within the bacterial cell. The central pathway in E.coli is the glycolysis and one of its key enzymes is the [http://www.genome.jp/dbget-bin/www_bget?ec:1.2.1.12 Glyceraldehyde 3-phosphate dehydrogenase (GAP-DH)]. As part of this reaction NAD<sup>+</sup> is reduced to NADH+H<sup>+</sup>. Thus this intermediate takes part in the electron flow needed for the generation of electricity. |
| - | The resulting amount of the intermediate NADH | + | The resulting amount of the intermediate NADH+H<sup>+</sup> can be calculated with the modeled Michaelis-Menten equation: <br> |
</p> | </p> | ||
| Line 89: | Line 89: | ||
| - | + | where ''v<sub>max</sub>'' is the maximum rate achieved by the system | |
| - | and '' | + | and ''K<sub>m</sub>'' describes the substrate concentration at which reaction rate is half-maximal.<br> |
| - | Further | + | Further ''v<sub>max</sub>'' can be also represented as: |
| Line 99: | Line 99: | ||
| - | + | where ''k<sub>cat</sub>'' represents the rate-limiting turnover number | |
and ''E0'' represents the enzyme concentration. | and ''E0'' represents the enzyme concentration. | ||
The resulting equation, alternative to (1), would be: | The resulting equation, alternative to (1), would be: | ||
| Line 108: | Line 108: | ||
<p align="justify"> | <p align="justify"> | ||
| - | The data of the kinetics | + | The data of the kinetics were obtained from the internet database [http://www.brenda-enzymes.org/php/result_flat.php4?ecno=1.2.1.12 BRENDA]. For ''E.coli'' the ''Km'' value for the NAD as a substrate is 0.045 for the wild-type enzyme. The ''k<sub>cat</sub>'' value for the ''E.coli'' for the wild-type is 0.268[1/s] ([http://abbs.oxfordjournals.org/content/44/6/527.long1 Errafiy, Soukri , 2012]). |
</p> | </p> | ||
| - | + | <br><br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <br> | + | |
===Simulation=== | ===Simulation=== | ||
The values ''k<sub>cat</sub>'' and ''K<sub>m</sub>'' obtained as mentioned above were applied in the simulation of the | The values ''k<sub>cat</sub>'' and ''K<sub>m</sub>'' obtained as mentioned above were applied in the simulation of the | ||
production of NADH. The start concentration of the enzyme GAP-DH was set at 10 µM and the simulation time span at 10 sec. | production of NADH. The start concentration of the enzyme GAP-DH was set at 10 µM and the simulation time span at 10 sec. | ||
| - | The simulation was performed for five different NAD<sup>+</sup> concentrations: 10 µM, | + | The simulation was performed for five different NAD<sup>+</sup> concentrations: 10 µM, 50 µM, 100 µM and 500 µM . |
The MATLAB source code can be obtained as .m file [https://static.igem.org/mediawiki/2013/5/56/Bielefeld-germany-model-inter-matlab-code.m here]. | The MATLAB source code can be obtained as .m file [https://static.igem.org/mediawiki/2013/5/56/Bielefeld-germany-model-inter-matlab-code.m here]. | ||
| - | Exemplary curves of the concentration change over time for the start NAD<sup>+</sup> concentration of 10 µM and 1 M are shown in figure 1 and figure 2 respectively. The curves showing the concentration change of the | + | Exemplary curves of the concentration change over time for the start NAD<sup>+</sup> concentration of 10 µM and 1 M are shown in figure 1 and figure 2 respectively. The curves showing the concentration change of the product NADH for all simulated start concentration of substrate are shown in the Figure 3. |
<br><br> | <br><br> | ||
[[File:Bielefeld-germany-model-inter-diag-2.jpg|500px|center|thumb|'''Figure 1''': The curve of concentration change for the substrate NAD<sup>+</sup> and product NADH . Start concentration of substrate set at 10 µM]] | [[File:Bielefeld-germany-model-inter-diag-2.jpg|500px|center|thumb|'''Figure 1''': The curve of concentration change for the substrate NAD<sup>+</sup> and product NADH . Start concentration of substrate set at 10 µM]] | ||
| Line 132: | Line 125: | ||
<br> | <br> | ||
| - | |||
| - | |||
[[File:Bielefeld-germany-model-inter-diag-all2.PNG|500px|center|thumb|'''Figure 3''': The curve of concentration change of the product NADH within 10 seconds for different substrate start concentrations [NAD].]] | [[File:Bielefeld-germany-model-inter-diag-all2.PNG|500px|center|thumb|'''Figure 3''': The curve of concentration change of the product NADH within 10 seconds for different substrate start concentrations [NAD].]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 145: | Line 131: | ||
==Mediator Reduction== | ==Mediator Reduction== | ||
<p align="justify"> | <p align="justify"> | ||
| - | In Microbial Fuel Cell microorganisms provide the electrons for the anode from the oxidation of the substrate(s) in the intracellular metabolic pathways. | + | In a Microbial Fuel Cell, microorganisms provide the electrons for the anode from the oxidation of the substrate(s) in the intracellular metabolic pathways. |
The transfer of the electrons to electrodes has been demonstrated for several species, though this process is inefficient as far as Coulombic yield and current generation are concerned [A]. | The transfer of the electrons to electrodes has been demonstrated for several species, though this process is inefficient as far as Coulombic yield and current generation are concerned [A]. | ||
| - | Hence, the application of | + | Hence, the application of electrochemical mediators is essential for the construction of a Microbial Fuel Cell. |
| - | Mediators are usually small water-soluble molecules, which are capable of undergoing the redox transformations. The mediator acts as an electron shuttle, enhancing the kinetics of the electron transfer. This approach has been proven to be generally quite successful and many substances were tested for their potential as electron | + | Mediators are usually small water-soluble molecules, which are capable of undergoing the redox transformations. The mediator acts as an electron shuttle, enhancing the kinetics of the electron transfer. This approach has been proven to be generally quite successful and many substances were tested for their potential as electron shuttles.<br> |
<br> | <br> | ||
| - | There are two classes of mediators, | + | There are two classes of mediators, endogenous and exogenous mediators. The endogenous mediators are generated by the bacteria and can be secreted to the medium and then be oxidized at the electrode. The exogenous mediators are redox molecules that are chemically synthesized and must be added into the anode chamber of the Microbial Fuel Cell in order to enable electron transfer from bacterial metabolic pathways to the anode. <br> |
<br> | <br> | ||
Therefore, the anodic performance dependents not only on the nature and the rate of the metabolism, but on the nature and the rate of electron transfer from the mediator to the anode as well. | Therefore, the anodic performance dependents not only on the nature and the rate of the metabolism, but on the nature and the rate of electron transfer from the mediator to the anode as well. | ||
| Line 158: | Line 144: | ||
* N-methyl phenazine (NMP)methosulfate, | * N-methyl phenazine (NMP)methosulfate, | ||
* 1-methoxy-5-methyl phenazine (MNMP) methosulfate and | * 1-methoxy-5-methyl phenazine (MNMP) methosulfate and | ||
| - | * Meldola Blue (MB ) | + | * Meldola Blue (MB) |
</p> | </p> | ||
<br><br><br><br> | <br><br><br><br> | ||
| Line 168: | Line 154: | ||
| - | The kinetic properties of the electrocatalytic oxidation of NADH and reduction of the soluble mediator, regarding rate constants were studied by cyclic voltammetry and chronoamperometry [ | + | The kinetic properties of the electrocatalytic oxidation of NADH and reduction of the soluble mediator, regarding rate constants were studied by cyclic voltammetry and chronoamperometry ([[Team:Bielefeld-Germany/Modelling/Reduction#References|Griindig ''et al''., 1995]]). |
The measured rates are shown in the table 1: | The measured rates are shown in the table 1: | ||
| - | [[File:Bielefeld-germany-model-reduction-table1.PNG|500px|center]] | + | [[File:Bielefeld-germany-model-reduction-table1.PNG|500px|center|thumb|Table 1: Kinetic characteristics for k<sub>2</sub> studied with cyclic voltammetry(CV) and chronoamperometry(CA) for the mediators:NMP, M-NMP and MB. The calculated rates obtained from ([[Team:Bielefeld-Germany/Modelling/Reduction#References|Griindig ''et al''., 1995]]]] |
| + | <br style="clear:both;"> | ||
| + | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
==Mediator Oxidation== | ==Mediator Oxidation== | ||
<p align="justify"> | <p align="justify"> | ||
| Line 198: | Line 182: | ||
''n'' is the number of electrons taking part in the electrode reaction,<br> | ''n'' is the number of electrons taking part in the electrode reaction,<br> | ||
F is the Faradays constant (96 500 C) and <br> | F is the Faradays constant (96 500 C) and <br> | ||
| - | k<sub>3</sub> is the reaction rate | + | k<sub>3</sub> is the reaction rate of the mediator oxidation at the anode.<br> |
</p> | </p> | ||
| Line 209: | Line 193: | ||
[[File:Bielefeld-germany-model-oxid-diagramm-MB-01.png|600px|center|thumb|'''Figure 1''': Curves for the current output, fluxes of concentration of the intermediates. Simulation performed for the start concentration of both NAD<sup>+</sup> and oxidized mediator '''MB''' at 100 µM.]] | [[File:Bielefeld-germany-model-oxid-diagramm-MB-01.png|600px|center|thumb|'''Figure 1''': Curves for the current output, fluxes of concentration of the intermediates. Simulation performed for the start concentration of both NAD<sup>+</sup> and oxidized mediator '''MB''' at 100 µM.]] | ||
<br><br> | <br><br> | ||
| - | Further simulation has been performed for four different start concentrations of the | + | Further simulation has been performed for four different start concentrations of the oxidized mediator in order to investigate how varying start concentration influence the current output. The concentrations were set at 10 µM, 50 µM, 100 µM and 500 µM. The time span was set at 30 sec. The curves of the current outputs are presented in the Figure 2. |
<br><br> | <br><br> | ||
[[File:Bielefeld-germany-model-oxid-diagramm-MB-02.png|600px|center|thumb|'''Figure 2''': Curves for the current output and fluxes of concentration of the intermediates. Simulation performed for the varying start concentration of the oxidized mediator '''MB''' [M<sub>ox</sub>] .]] | [[File:Bielefeld-germany-model-oxid-diagramm-MB-02.png|600px|center|thumb|'''Figure 2''': Curves for the current output and fluxes of concentration of the intermediates. Simulation performed for the varying start concentration of the oxidized mediator '''MB''' [M<sub>ox</sub>] .]] | ||
| Line 217: | Line 201: | ||
| - | Analogous simulations have been performed for the mediator '''NMP'''. As the reaction rate ''k<sub>3</sub>'' specific for this mediator could not be | + | Analogous simulations have been performed for the mediator '''NMP'''. As the reaction rate ''k<sub>3</sub>'' specific for this mediator could not be obtained, the value specific for the mediator '''MB''' was applied. The diagrams presenting the results of those simulations are shown in the Figure 3 and 4 respectively. |
<br><br> | <br><br> | ||
[[File:Bielefeld-germany-model-oxid-diagramm-NMP-03.png|600px|center|thumb|'''Figure 3''': Curves for the current output, fluxes of concentration of the intermediates. Simulation performed for the start concentration of both NAD<sup>+</sup> and oxidized mediator '''NMP''' at 100 µM.]] | [[File:Bielefeld-germany-model-oxid-diagramm-NMP-03.png|600px|center|thumb|'''Figure 3''': Curves for the current output, fluxes of concentration of the intermediates. Simulation performed for the start concentration of both NAD<sup>+</sup> and oxidized mediator '''NMP''' at 100 µM.]] | ||
| Line 227: | Line 211: | ||
</p> | </p> | ||
| + | |||
| + | <br> | ||
===Discussion=== | ===Discussion=== | ||
<p align="justify"> | <p align="justify"> | ||
| - | The curve for the current output simulated for the mediator '''MB''' has been presented in the | + | The curve for the current output simulated for the mediator '''MB''' has been presented in the Figure 1. It could be shown that the maximal output is reached rapidly after the start of the reaction system. Immediately after reaching the maximum, it decreases equally fast. At the 20 second mark it reaches a stable phase of about 20 per cent of the maximal current output.<br> |
| - | The comparison of different start concentrations of the oxidized mediator can be obtained from the | + | The comparison of different start concentrations of the oxidized mediator can be obtained from the Figure 2. The curve form is identical for all four concentrations and the reached current values differ only slightly for the different simulations. Also the maximum output is almost identical and is reached at the same time for all four approaches. |
Interestingly the curves obtained from the simulation for the mediator '''NMP''' are very similar to those for the mediator '''MB'''. This points that the reaction rate ''k<sub>3</sub>'' for the oxidation of the mediator at the anode plays a predominant role in generating current.<br> | Interestingly the curves obtained from the simulation for the mediator '''NMP''' are very similar to those for the mediator '''MB'''. This points that the reaction rate ''k<sub>3</sub>'' for the oxidation of the mediator at the anode plays a predominant role in generating current.<br> | ||
| - | Obviously | + | Obviously it is necessary to constantly provide the system with the redox molecules in order to sustain the current at the high level.This builds a strong case for the usse of endogenous mediators that are supplied and regenerated by the bacteria themselves. |
Another interesting aspect emerges when comparing the modeled current output curves and those obtained with the values measured in MFC. The measurements were performed in a self-designed MFC where only M9 medium and no bacteria was present in the anode chamber.The resulting curve has been shown in the figure 5. The mediator was added to the anode chamber only once at the beginning of the measurements. | Another interesting aspect emerges when comparing the modeled current output curves and those obtained with the values measured in MFC. The measurements were performed in a self-designed MFC where only M9 medium and no bacteria was present in the anode chamber.The resulting curve has been shown in the figure 5. The mediator was added to the anode chamber only once at the beginning of the measurements. | ||
| Line 239: | Line 225: | ||
<br> | <br> | ||
The resulting curve form is very similar to the curves observed in the models. There are though significant differences in the dimensions of both current/voltage and time span in which the reactions take place. | The resulting curve form is very similar to the curves observed in the models. There are though significant differences in the dimensions of both current/voltage and time span in which the reactions take place. | ||
| - | Those differences might result from the fact that in our model we did not take the resistance into consideration. | + | Those differences might result from the fact that in our model we did not take the resistance into consideration. |
| - | + | ||
| - | + | ||
| + | </p> | ||
<br><br> | <br><br> | ||
==Optimal Conditions== | ==Optimal Conditions== | ||
Latest revision as of 04:00, 29 October 2013
Modeling - Overview
Approach
In a Microbial Fuel Cell (MFC) the chemical energy is transformed into the electrical energy via a cascade of electrochemical reactions. Electrons are produced in the metabolic pathways and can be extracted from the cell and concentrated at the electrode by the electric potential differences. Alternatively the electrons can be transferred to the oxidized mediator molecules that transfer them further to the electrode. There is a variety of parameters and interactions that influence electricity generation. Therefore, there is the need to identify the bottleneck reactions and limiting factors. This approach reduces the complexity of the analysis and can give a deeper insight on the most important processes involved in the electricity generation.
In our theoretical analysis the focus was set to three bottleneck reactions involved in the electron flow from the metabolism of the bacterial cells to the cathode:
- Generation of intermediates NADH/H+ in the metabolic pathway of E.coli
- Reduction of oxidized mediators via the intermediate NADH
- Transfer of the electrons from reduced mediator to the electrode
Intermediates
The electrons that will be eventually transferred to the anode are generated during the biochemical processes within the bacterial cell. The central pathway in E.coli is the glycolysis and one of its key enzymes is the [http://www.genome.jp/dbget-bin/www_bget?ec:1.2.1.12 Glyceraldehyde 3-phosphate dehydrogenase (GAP-DH)]. As part of this reaction NAD+ is reduced to NADH+H+. Thus this intermediate takes part in the electron flow needed for the generation of electricity.
The resulting amount of the intermediate NADH+H+ can be calculated with the modeled Michaelis-Menten equation:
where vmax is the maximum rate achieved by the system
and Km describes the substrate concentration at which reaction rate is half-maximal.
Further vmax can be also represented as:
where kcat represents the rate-limiting turnover number
and E0 represents the enzyme concentration.
The resulting equation, alternative to (1), would be:
The data of the kinetics were obtained from the internet database [http://www.brenda-enzymes.org/php/result_flat.php4?ecno=1.2.1.12 BRENDA]. For E.coli the Km value for the NAD as a substrate is 0.045 for the wild-type enzyme. The kcat value for the E.coli for the wild-type is 0.268[1/s] ([http://abbs.oxfordjournals.org/content/44/6/527.long1 Errafiy, Soukri , 2012]).
Simulation
The values kcat and Km obtained as mentioned above were applied in the simulation of the
production of NADH. The start concentration of the enzyme GAP-DH was set at 10 µM and the simulation time span at 10 sec.
The simulation was performed for five different NAD+ concentrations: 10 µM, 50 µM, 100 µM and 500 µM .
The MATLAB source code can be obtained as .m file here.
Exemplary curves of the concentration change over time for the start NAD+ concentration of 10 µM and 1 M are shown in figure 1 and figure 2 respectively. The curves showing the concentration change of the product NADH for all simulated start concentration of substrate are shown in the Figure 3.
Mediator Reduction
In a Microbial Fuel Cell, microorganisms provide the electrons for the anode from the oxidation of the substrate(s) in the intracellular metabolic pathways.
The transfer of the electrons to electrodes has been demonstrated for several species, though this process is inefficient as far as Coulombic yield and current generation are concerned [A].
Hence, the application of electrochemical mediators is essential for the construction of a Microbial Fuel Cell.
Mediators are usually small water-soluble molecules, which are capable of undergoing the redox transformations. The mediator acts as an electron shuttle, enhancing the kinetics of the electron transfer. This approach has been proven to be generally quite successful and many substances were tested for their potential as electron shuttles.
There are two classes of mediators, endogenous and exogenous mediators. The endogenous mediators are generated by the bacteria and can be secreted to the medium and then be oxidized at the electrode. The exogenous mediators are redox molecules that are chemically synthesized and must be added into the anode chamber of the Microbial Fuel Cell in order to enable electron transfer from bacterial metabolic pathways to the anode.
Therefore, the anodic performance dependents not only on the nature and the rate of the metabolism, but on the nature and the rate of electron transfer from the mediator to the anode as well.
Several exogenous mediators have been already studied in regard to their effect on electron transfer and so their impact on the electricity generation. Among those mediators are methylene blue (MB), neutral red (NR), thionin, ferricyanide, humic acid or methyl viologen.
In our model we modelled the influence of the three of the mediators:
- N-methyl phenazine (NMP)methosulfate,
- 1-methoxy-5-methyl phenazine (MNMP) methosulfate and
- Meldola Blue (MB)
Reaction Kinetics
In contrast to the first bottle neck reaction of our model, which has been described as the Michaelis-Menten reaction, the redox reaction between the bacterial metabolites and the oxidized mediator can be considered as a first order reaction:
The kinetic properties of the electrocatalytic oxidation of NADH and reduction of the soluble mediator, regarding rate constants were studied by cyclic voltammetry and chronoamperometry (Griindig et al., 1995).
The measured rates are shown in the table 1:

Mediator Oxidation
In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation:
,where Mred is the reduced mediator and
Mox the oxidized mediator.
Then the current output can be calculated based on formula according to the Faraday's law:
,where I is the current density [A]
[Mred] is the concentration of reduced mediator in the chamber
n is the number of electrons taking part in the electrode reaction,
F is the Faradays constant (96 500 C) and
k3 is the reaction rate of the mediator oxidation at the anode.
Simulation
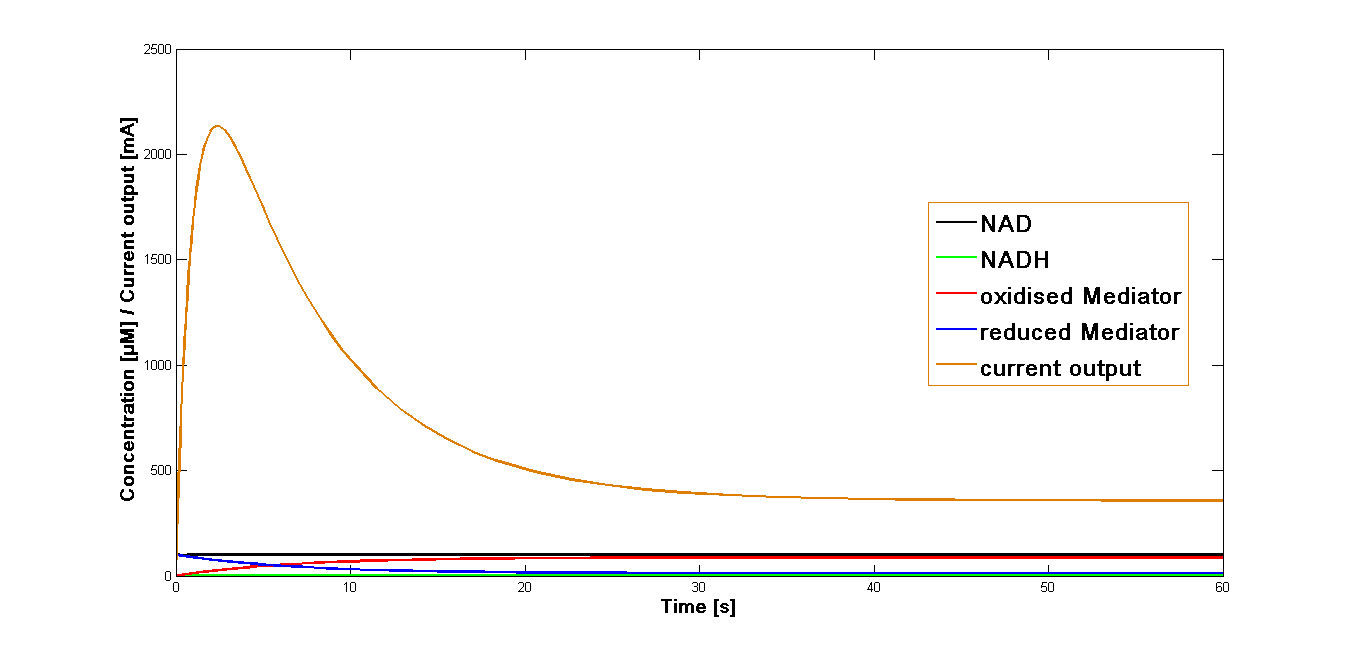
The value k3 obtained as mentioned above was applied in the simulation of the current output in the final reaction of the three-reaction model. The start concentrations of the NAD+ and oxidized mediator were set as in the simulations preformed for the proceeding reactions at 100 µM. The simulation time span was set for 60 sec.
The resulting plot for the mediator MB is shown in the Figure 1, below:
Further simulation has been performed for four different start concentrations of the oxidized mediator in order to investigate how varying start concentration influence the current output. The concentrations were set at 10 µM, 50 µM, 100 µM and 500 µM. The time span was set at 30 sec. The curves of the current outputs are presented in the Figure 2.
The MATLAB source code for both simulations can be obtained here and here.
Analogous simulations have been performed for the mediator NMP. As the reaction rate k3 specific for this mediator could not be obtained, the value specific for the mediator MB was applied. The diagrams presenting the results of those simulations are shown in the Figure 3 and 4 respectively.
The according .m files with MATLAB source code can be downloaded from here and here.
Discussion
The curve for the current output simulated for the mediator MB has been presented in the Figure 1. It could be shown that the maximal output is reached rapidly after the start of the reaction system. Immediately after reaching the maximum, it decreases equally fast. At the 20 second mark it reaches a stable phase of about 20 per cent of the maximal current output.
The comparison of different start concentrations of the oxidized mediator can be obtained from the Figure 2. The curve form is identical for all four concentrations and the reached current values differ only slightly for the different simulations. Also the maximum output is almost identical and is reached at the same time for all four approaches.
Interestingly the curves obtained from the simulation for the mediator NMP are very similar to those for the mediator MB. This points that the reaction rate k3 for the oxidation of the mediator at the anode plays a predominant role in generating current.
Obviously it is necessary to constantly provide the system with the redox molecules in order to sustain the current at the high level.This builds a strong case for the usse of endogenous mediators that are supplied and regenerated by the bacteria themselves.
Another interesting aspect emerges when comparing the modeled current output curves and those obtained with the values measured in MFC. The measurements were performed in a self-designed MFC where only M9 medium and no bacteria was present in the anode chamber.The resulting curve has been shown in the figure 5. The mediator was added to the anode chamber only once at the beginning of the measurements.
The resulting curve form is very similar to the curves observed in the models. There are though significant differences in the dimensions of both current/voltage and time span in which the reactions take place.
Those differences might result from the fact that in our model we did not take the resistance into consideration.
Optimal Conditions
The Microbial Fuel Cell will be subjected to numerous parameters, which will heavily influence its performance. Therefore, there is a need to inquiry the impact of those parameters on the efficiency of the system. In our approach we focused on how the reduction potential of the given mediators depends on two parameters, namely the pH value and the temperature. All values were calculated based on the [http://en.wikipedia.org/wiki/Nernst_equation Nernst equation], which describes the relation between the electromotive force of the full cell and the temperature or the pH-value:
Optimal pH value
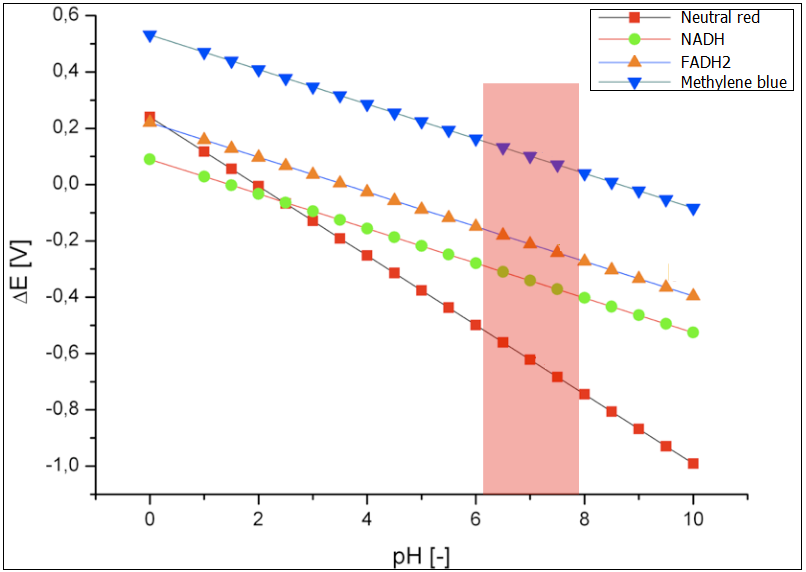
The following exogenous mediators were analyzed under varying pH-value: Neutral red, NADH, FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of E.coli have been marked in red. Methylene blue has been chosen as the proof of concept for the experimental examination of the fuel cell, after various initial tests. Hence, it has been in the focus of the modeling of the performance as well. The reduction potential for the optimal growth conditions for E.coli, is between ~0.13 and ~0.8 V. The reduction potential for neutral red lies in this pH interval is between -0.4 and -0.7. This value is too low and would not lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2.
Optimal temperature
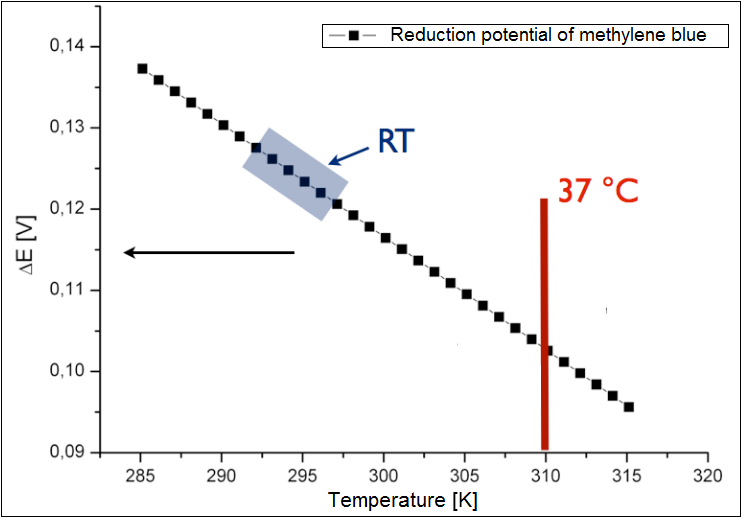
The analysis was conducted only for an exogenous mediator methylene blue. The results have been shown in the figure 2 below. It could be shown, that the calculated reduction potential differs only slightly for the broad spectrum of the temperature, and all lies at about 0.1 V. Therefor the temperature is not the limiting parameter for the performance of the fuel cell. The charge at the optimal growth condition for E. coli (37 °C or 310 K) is ~0.13 .
Combined calculations for the proof system
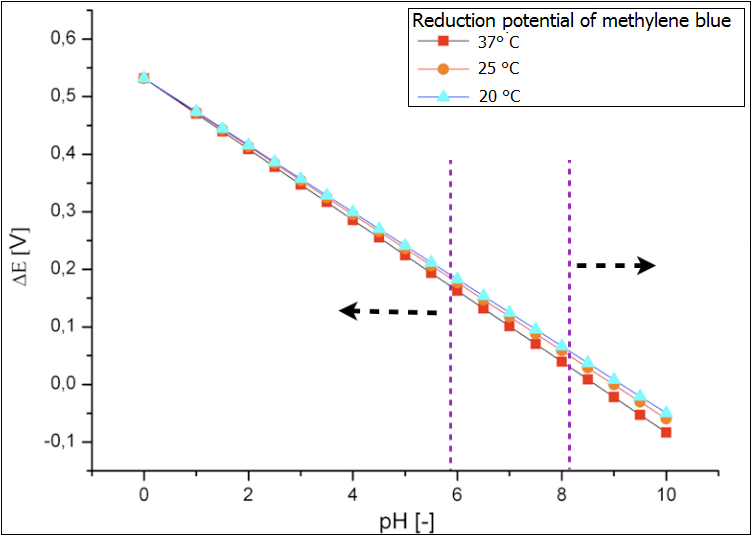
Ultimately the analysis of the influence of both temperature and pH value on the reduction potential of the proof system methylene blue has been performed. Its results are depicted in the figure 3. Again it could be shown that the temperature does not affect the reduction potential and therefore the effectiveness of the fuel cell. In contrast the pH-value in the chamber should be monitored and kept at the values that correspond to the optimal growth condition of the E.coli cells. The values below 6 would lead to too high reduction potential via the accumulation of the fermentation products under anaerob cultivation and decreased efficiency. Too basic conditions in the chamber (above 8) would lead to oxidation of the glucose and to so-called Blue-Bottle-Effect. The performance of the fuel cell would be also worse.
References
- Errafiy N, Soukri A (2012). Purification and partial characterization of glyceraldehyde-3-phosphate dehydrogenase from the ciliate Tetrahymena thermophila. [http://abbs.oxfordjournals.org/content/44/6/527.long Acta Biochimica et Biophysica Sinica, Volume 44](6), 527-534.
 "
"