Team:Paris Saclay/Notebook/August/22
From 2013.igem.org
(→2 - Electrophoresis of PCR product : BphR2 Part I) |
|||
| (21 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
==='''A - Aerobic/Anaerobic regulation system'''=== | ==='''A - Aerobic/Anaerobic regulation system'''=== | ||
| - | ===='''Objective : characterize | + | ===='''Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== |
| - | ===='''1 - Plasmid extraction of | + | ===='''1 - Plasmid extraction of BBa_K1155000 from DH5α'''==== |
Nguyen | Nguyen | ||
| - | Protocol : [[Team:Paris_Saclay/ | + | Protocol : [[Team:Paris_Saclay/extraction|High-copy plamid extraction]] |
Nanodrop : | Nanodrop : | ||
| - | * | + | * BBa_K1155000 : 175ng/µL |
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | + | The extraction was good. We will digested the plasmid. | |
|} | |} | ||
| - | ===='''2 - Digestion of | + | ===='''2 - Digestion of BBa_K1155000 by SpeI'''==== |
| + | |||
| - | |||
Used quantities : | Used quantities : | ||
| - | * | + | * BBa_K1155000 : 10µL |
* Buffer FD : 2µL | * Buffer FD : 2µL | ||
| - | * | + | * SpeI : 2µL |
* H2O : 6 µL | * H2O : 6 µL | ||
| - | We let digestions at 37°C during 10 minutes | + | We let digestions at 37°C during 10 minutes. |
| - | + | ||
| - | + | ||
| - | + | ===='''Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in pSB3K3'''==== | |
| - | ===='''1 - Gel purification of the digestion of | + | ===='''1 - Gel purification of the digestion of BBa_J04450 by EcoRI/PstI '''==== |
XiaoJing | XiaoJing | ||
| - | Protocol : [ | + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] |
Nanodrop : | Nanodrop : | ||
| - | * | + | * pSB3K3 : 4ng/µL |
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | The Nanodrop gives us a very few quantity of | + | The Nanodrop gives us a very few quantity of pSB3K3 so we decided to check it with a first electrophoresis. |
|} | |} | ||
| + | |||
| + | ===='''2 - Electrophoresis of gel purification of the digestion of BBa_J04450 by EcoRI/PstI '''==== | ||
| + | |||
| + | XiaoJing | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |]] | + | | style="width:350px;border:1px solid black;" |[[File:Psgel12208.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL DNA Ladder | * Well 1 : 6µL DNA Ladder | ||
| - | * Well 2 : 5µL of | + | * Well 2 : 5µL of BBa_J04450 digested by EcoRI/Pst I + 1µl of 6X loading dye |
* Gel : 1% | * Gel : 1% | ||
|} | |} | ||
Expect sizes : | Expect sizes : | ||
| - | * | + | * pSB3K3 : 2750 bp |
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We can see anything with the fisrt electrophoresis that's why we made an EtOH precipitation. | + | We can't see anything with the fisrt electrophoresis that's why we made an EtOH precipitation. |
|} | |} | ||
| - | + | ===='''3 - Ethanol precipitation of the digestion of BBa_J04450 by EcoRI/PstI '''==== | |
| - | We used 34µL of DNA. | + | Protocol : [[Team:Paris_Saclay/ethanol|EtOH precipitation]] |
| + | |||
| + | We used 34µL of DNA. | ||
| Line 89: | Line 93: | ||
* dNTP : 1µL | * dNTP : 1µL | ||
* Phusion : 1µL | * Phusion : 1µL | ||
| - | * | + | * DMS0 : 2µL |
* H2O : 31µL | * H2O : 31µL | ||
| Line 101: | Line 105: | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |]] | + | | style="width:350px;border:1px solid black;" |[[File:Psgel22208.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL DNA Ladder | * Well 1 : 6µL DNA Ladder | ||
| Line 109: | Line 113: | ||
Expected sizes : | Expected sizes : | ||
| - | + | * BphR2 Part I : 178 kb | |
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We obtained fragments at the right size. We can purify it. |
|} | |} | ||
| Line 120: | Line 124: | ||
Damir | Damir | ||
| - | Protocol : [ | + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] |
Nanodrop : | Nanodrop : | ||
| Line 126: | Line 130: | ||
* RBS-BphR2 Part I, tube 2 : 75ng/µL | * RBS-BphR2 Part I, tube 2 : 75ng/µL | ||
| - | {| | + | |
| - | + | {| border="1" align="center" | |
| - | + | |[[Team:Paris Saclay/Notebook/August/21|<big>Previous day</big>]] | |
| + | |||
| + | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| + | |||
| + | |[[Team:Paris Saclay/Notebook/August/23|<big>Next day</big>]] | ||
|} | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 01:16, 5 October 2013
Notebook : August 22
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - Plasmid extraction of BBa_K1155000 from DH5α
Nguyen
Protocol : High-copy plamid extraction
Nanodrop :
- BBa_K1155000 : 175ng/µL
|
The extraction was good. We will digested the plasmid. |
2 - Digestion of BBa_K1155000 by SpeI
Used quantities :
- BBa_K1155000 : 10µL
- Buffer FD : 2µL
- SpeI : 2µL
- H2O : 6 µL
We let digestions at 37°C during 10 minutes.
Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in pSB3K3
1 - Gel purification of the digestion of BBa_J04450 by EcoRI/PstI
XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- pSB3K3 : 4ng/µL
|
The Nanodrop gives us a very few quantity of pSB3K3 so we decided to check it with a first electrophoresis. |
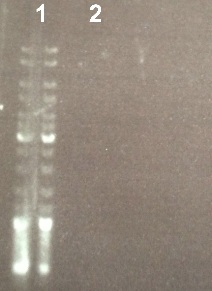
2 - Electrophoresis of gel purification of the digestion of BBa_J04450 by EcoRI/PstI
XiaoJing
|
|
Expect sizes :
- pSB3K3 : 2750 bp
|
We can't see anything with the fisrt electrophoresis that's why we made an EtOH precipitation. |
3 - Ethanol precipitation of the digestion of BBa_J04450 by EcoRI/PstI
Protocol : EtOH precipitation
We used 34µL of DNA.
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining BphR2 protein
1 - PCR of BphR2 Part I
Damir
Used quantities :
- Oligo 54F : 2µL
- Oligo 55R : 2µL
- DNA : 1µL
- Buffer Phusion : 10µL
- dNTP : 1µL
- Phusion : 1µL
- DMS0 : 2µL
- H2O : 31µL
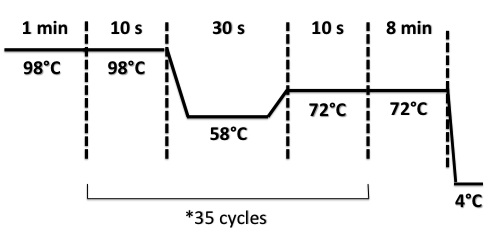
PCR program :
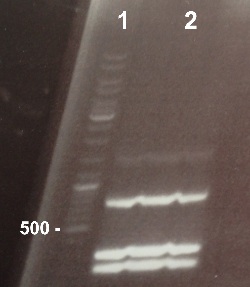
2 - Electrophoresis of PCR product : BphR2 Part I
Damir
|
|
Expected sizes :
- BphR2 Part I : 178 kb
|
We obtained fragments at the right size. We can purify it. |
3 - Gel purification of PCR product : BphR2 Part I
Damir
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- RBS-BphR2 Part I, tube 1 : 42ng/µL
- RBS-BphR2 Part I, tube 2 : 75ng/µL
| Previous day | Back to calendar | Next day |
 "
"