Team:Bielefeld-Germany/Project/Porins
From 2013.igem.org
| (54 intermediate revisions not shown) | |||
| Line 9: | Line 9: | ||
<html> | <html> | ||
<style> | <style> | ||
| - | h1{ | + | h1{margin-top:70px; } |
| - | + | ||
#globalwrapper ul {padding-left:40px; padding-right:40px;} | #globalwrapper ul {padding-left:40px; padding-right:40px;} | ||
| Line 56: | Line 56: | ||
<a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Porins#Theory">Theory</a></div> | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Porins#Theory">Theory</a></div> | ||
<div class="bigbutton"> | <div class="bigbutton"> | ||
| - | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Porins# | + | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Porins#Genetic_Approach">Genetic Approach</a></div> |
<div class="bigbutton"> | <div class="bigbutton"> | ||
<a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Porins#Results">Results</a></div> | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Porins#Results">Results</a></div> | ||
| Line 62: | Line 62: | ||
</div> | </div> | ||
</html> | </html> | ||
| + | |||
| Line 67: | Line 68: | ||
| - | [[Image:Bielefeld-germany- | + | [[Image:Bielefeld-germany-project2-overview-porine.png|300px|left|thumb|<p align="justify">'''Figure 1: Schematic overview of the enhancement mechanism of electron shuttle-mediated electron transfer between bacteria and the anode of MFCs by the porin OprF. Oxidized mediators diffuse into the periplasmatic space where they accept electrons. In turn, reduced mediators diffuse through outer membrane porins and donate their electrons to the electrode.'''</p>]] |
<p align="justify"> | <p align="justify"> | ||
| - | The Microbial Fuel Cell (MFC) can be a future | + | The Microbial Fuel Cell (MFC) can be a future environmentally friendly biotechnological application for the production of electrical energy. As a future alternative energy source, the bioelectricity generation must become more efficient. A major limiting factor is the low permeability of the bacterial membrane, hindering transport of electron shuttles through the membrane and thereby restricting the electron shuttle-mediated extracellular electron transfer (EET) from bacteria to electrodes. This results in a reduced electrical power output of the MFC. Therefore, we heterologously expressed the porin protein OprF from ''Pseudomonas fluorescens'' in ''Escherichia coli''. This leads to a much higher current output in comparison to its parental strain (''E. coli'' KRX). This is most likely caused by improved electron shuttle-mediated extracellular electron transfer through dramatically increased membrane permeability. The heterologous expression of the outer membrane porin OprF from ''Pseudomonas fluorescens'' in ''Escherichia coli'' is a great genetic strategy to overcome limitations due to the membrane and to increase electricity generation by microorganisms. |
</p> | </p> | ||
| Line 81: | Line 82: | ||
*<p align="justify">The efficiency of extracellular electron transfer is a major limiting factor for electricity power output of MFCs. The electron shuttle-mediated EET is the most common EET pathway for microorganisms in a Microbial Fuel Cell such as ''Escherichia coli'' ([[Team:Bielefeld-Germany/Project/Porins#References|Logan, 2009]]).</p> | *<p align="justify">The efficiency of extracellular electron transfer is a major limiting factor for electricity power output of MFCs. The electron shuttle-mediated EET is the most common EET pathway for microorganisms in a Microbial Fuel Cell such as ''Escherichia coli'' ([[Team:Bielefeld-Germany/Project/Porins#References|Logan, 2009]]).</p> | ||
| - | *<p align="justify">The cell membrane is a natural protective layer enabling proper physiology of bacteria, but it is also a barrier for | + | *<p align="justify">The cell membrane is a natural protective layer enabling proper physiology of bacteria, but it is also a barrier for substrate exchange, because an efficient electron shuttle-mediated EET requires diffusion of shuttle molecules across the cell membrane (Figure 1). However, the bacterial outer membrane is a low permeable barrier for the transport of electron shuttles across the cell membrane, which limits among other things the efficiency of electron transport and is responsible for the low power output of MFCs up to now. One strategy to improve the electron shuttle mediated EET is to enhance the permeability of the cell membrane. Evolutionary strategies, e.g. application of a continuous cell stress, are very time-consuming and unpredictable. The chemical treatment with permeabilizers can perforate the outer membrane but has negative impact on the viability and metabolism of the cells. ([[Team:Bielefeld-Germany/Project/Porins#References|Liu ''et al''., 2012]])</p> |
| - | *<p align="justify">We thus hypothesize that genetic engineering of a highly permeable cell membrane would be | + | *<p align="justify">We thus hypothesize that genetic engineering of a highly permeable cell membrane would be beneficial. Therefore, we introduce pore forming proteins in the outer membrane. These so called porins are common bacterial outer membrane proteins, which can form water-filled channels across the membrane of Gram-negative bacteria. They allow hydrophilic substances to diffuse across the outer membrane ([[Team:Bielefeld-Germany/Project/Porins#References|Hancock and Brinkman, 2002]]).</p> |
| - | *<p align="justify">''E. coli'' expresses different | + | *<p align="justify">''E. coli'' expresses different porines, for example OmpF and OmpC. But these natural occurring porines are only permeable for molecules smaller than 600 Da, which decreases the range of usable mediators and the mediator transport kinetics ([[Team:Bielefeld-Germany/Project/Porins#References|Yong ''et al''., 2013]]). Thus, we thought about enhancing the amount and size of pores in the outer cell membrane with much larger porins than those of ''E. coli''.</p> |
| - | *<p align="justify">OprF is a major outer membrane protein in ''Pseudomonas | + | *<p align="justify">OprF is a major outer membrane protein in ''Pseudomonas'' species, which acts as a non-specific porin protein and adhesin. OprF represents one of the largest pore sizes on bacterial outer membranes, allowing diffusion of polysaccharides in a range of 2000 to 3000 Da. In contrast to OprF, the general porin channels of ''E. coli'' are only permeable to sugars with sizes smaller than 600 Da. ([[Team:Bielefeld-Germany/Project/Porins#References|Yong ''et al''., 2013]])</p> |
| - | *<p align="justify">In due consideration of all facts, heterologous expression of the porin protein OprF from ''Pseudomonas fluorescens'' in ''E. coli'' will improve the EET between bacteria and electrodes by increase of membrane permeability. A heterologous expression of large porins improves besides mediator | + | *<p align="justify">In due consideration of all facts, heterologous expression of the porin protein OprF from ''Pseudomonas fluorescens'' in ''E. coli'' will improve the EET between bacteria and electrodes by increase of membrane permeability. A heterologous expression of large porins improves, besides mediator efficiency, the spectrum of usable mediators. Thus, alternative, environmentally friendly mediators such as NADH and riboflavin might become applicable instead of the artificial systems used up to now.</p> |
==Genetic Approach== | ==Genetic Approach== | ||
| - | *<p align="justify">The | + | *<p align="justify">The ''oprF'' gene from ''Pseudomonas fluorescens'' was cloned and heterologously expressed in ''Escherichia coli'' KRX under the control of different promoters (Table 1).</p> |
| - | [[Image:Table1_Overview_OprF_Devices.jpg|300px|thumb|left|<p align="justify"> '''Table 1: Overview of OprF devices. Combination of OprF coding BioBrick (<bbpart>BBa_K1172501</bbpart>) with different | + | [[Image:Table1_Overview_OprF_Devices.jpg|300px|thumb|left|<p align="justify"> '''Table 1: Overview of OprF devices. Combination of OprF coding BioBrick (<bbpart>BBa_K1172501</bbpart>) with different promoters and RBS. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_PSB1C3_OprF.jpg|300px|thumb|center|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_PSB1C3_OprF.jpg|300px|thumb|center|<p align="justify"> '''Figure 2: pSB1C3 – <bbpart>BBa_K1172501</bbpart> OprF BioBrick (1298 bp) was examined by [[Team:Bielefeld-Germany/Labjournal/Molecular#Restriction analysis|restriction analysis]] and [https://scf.cebitec.uni-bielefeld.de sequencing]. '''</p>]] |
| Line 100: | Line 101: | ||
| - | *<p align="justify">Upon | + | *<p align="justify">Upon expression of the outer membrane porin protein OprF, the morphological and physicochemical characteristics of the ''E. coli'' surface were analyzed. [[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) | SDS-PAGE]] combined with [[Team:Bielefeld-Germany/Labjournal/Molecular#MALDI-TOF|MALDI-TOF MS/MS]], different membrane permeability assays ([[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay|NPN]] and [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay | ONPG]]), a [[Team:Bielefeld-Germany/Labjournal/Molecular#Hexadecan Assay|hydrophobicity assay]] and [[Team:Bielefeld-Germany/Labjournal/Molecular#Preparing samples for Atomic Force Microscopy (AFM) analysis of the OM of cells|Atomic Force Microscopy]] (AFM) characterize the OprF BioBrick <bbpart>BBa_K1172501</bbpart>.</p> |
| Line 106: | Line 107: | ||
| - | *<p align="justify">OprF | + | *<p align="justify">OprF was detected in an [[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)|SDS-PAGE]] with a broad overexpression band. Protein isolation of the outer membrane porin OprF by release of periplasmic protein fraction from ''E. coli'' via [[Team:Bielefeld-Germany/Labjournal/Molecular#Cold osmotic shock|cold osmotic shock]] using [[Team:Bielefeld-Germany/Labjournal/Molecular#Cell Fractioning Buffers | Cell fractionating buffer 2.3]] was successful.</p> |
| - | [[Image: | + | [[Image:SDS PAGE OprF4 0,2%SDS.jpg|300px|thumb|left|<p align="justify"> '''Figure 3: [[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)|SDS-PAGE]] with [http://www.thermoscientific.com/ecomm/servlet/productsdetail_11152___13576050_-1 Prestained Protein Ladder from Thermo Scientific] as marker. Comparison of protein expression between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart> and <bbpart>BBa_K1172507</bbpart> after [[Team:Bielefeld-Germany/Labjournal/Molecular#Cold osmotic shock | periplasmic protein fractioning]] with [[Team:Bielefeld-Germany/Labjournal/Molecular#Cell Fractioning Buffers | Cell fractionating buffer 2.3]]. The band used for the [[Team:Bielefeld-Germany/Labjournal/Molecular#MALDI-TOF|MALDI-TOF MS/MS]] is marked with an arrow. '''</p>]] |
| - | *<p align="justify">The [[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)|SDS-PAGE]] shows a significantly higher protein concentration | + | *<p align="justify">The [[Team:Bielefeld-Germany/Labjournal/Molecular#Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)|SDS-PAGE]] shows a significantly higher protein concentration in extracts from ''E.coli'' expressing OprF from a T7 promoter (<bbpart>BBa_K1172502</bbpart>). This is most likely caused by the higher membrane permeability (shown with [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay|NPN]] and [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG]] uptake assay), allowing an increased release of membrane proteins by 0.2 % SDS. Nevertheless, a strong overexpression band can be observed at the expected OprF size of about 36 kDa for <bbpart>BBa_K1172502</bbpart>, which is equated with a strong expression and overproduction of OprF.</p> |
| - | *<p align="justify">Furthermore we were able to identify the overexpressed outer membrane porin (Figure. | + | *<p align="justify">Furthermore we were able to identify the overexpressed outer membrane porin (Figure. 3) with [[Team:Bielefeld-Germany/Labjournal/Molecular#MALDI-TOF|MALDI-TOF MS/MS]].</p> |
| - | **<p align="justify">[[Team:Bielefeld-Germany/Labjournal/Molecular#Tryptic digest of gel lanes for analysis with MALDI-TOF | Tryptic digest]] of the gel | + | **<p align="justify">[[Team:Bielefeld-Germany/Labjournal/Molecular#Tryptic digest of gel lanes for analysis with MALDI-TOF | Tryptic digest]] of the gel band thought to represent OprF and analysis with [[Team:Bielefeld-Germany/Labjournal/Molecular#MALDI-TOF|MALDI-TOF]] confirmed the outer membrane porin with a Mascot Score of 222 against the bacteria database.</p> |
| + | |||
| + | |||
| + | ===Growth characteristics=== | ||
| + | |||
| + | |||
| + | *<p align="justify">In order to test the effect of the protein expression on the cell growth, growth was measured as increase in optical density and plotted against time.</p> | ||
| + | [[Image:iGEM_Bielefeld_Growth_OprF.jpg|400px|thumb|left|<p align="justify"> '''Figure 4: Growth curves for ''E. coli'' KRX with [[Team:Bielefeld-Germany/Labjournal/Molecular#LB medium|LB medium]]. Comparison between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with ''oprF'' plasmids <bbpart>BBa_K1172501</bbpart>, <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart> and <bbpart>BBa_K1172507</bbpart>. ''oprF'' gene expression was induced for inducable promoters at OD<sub>600</sub> = 1.0. All data are representing two biological and two technical replicates with standard deviation. '''</p>]] | ||
| + | *<p align="justify">''E. coli'' KRX wild type shows the best growth characteristics with a maximal optical density OD<sub>600</sub> = 4.0. ''E. coli'' KRX with ''oprF'' coding plasmid shows a slightly lower growth due to the higher replication stress. ''E. coli'' KRX expressing ''oprF'' under control of strong Anderson and ''lac'' promoters are showing 10% decreased maximal cell density and ''E. coli'' KRX expressing ''oprF'' under control of T7 promoter shows a further decreased growth which was 45% reduced in comparison to ''E. coli'' KRX wild type. This lower growth is based on an increased expression of ''oprF'' on the cellular surface and therefore on higher metabolic pressure, which was confirmed with different assays, which can be found in the further characterization of the outer membrane protein OprF.</p> | ||
| Line 120: | Line 129: | ||
| - | *<p align="justify">The heterologous expression of the outer membrane porin OprF will enhance the hydrophobicity of cell membrane. The outward-facing side groups on each of the β-strands of the OprF monomer are hydrophobic. Therefore | + | *<p align="justify">The heterologous expression of the outer membrane porin OprF will enhance the hydrophobicity of cell membrane. The outward-facing side groups on each of the β-strands of the OprF monomer are hydrophobic. Therefore, heterologous expression should be detectable by an increase in hydrophobicity. The [[Team:Bielefeld-Germany/Labjournal/Molecular#Hexadecan Assay | Hexadecane-Hydrophobicity-Assay]] is based on different affinities to hexadecane of dissimilar cell-types according to their cell surface. Hydrophobicity can be observed by specific changes in OD<sub>600</sub> of the aqueous phase.</p> |
| - | *<p align="justify">An increasing hydrophobicity of cell membrane changes the physicochemical properties of the cell. This could effect for example the cell-electrode interaction. Therefore, we investigated the cellular surface characteristic by comparing ''Escherichia coli'' KRX | + | *<p align="justify">An increasing hydrophobicity of cell membrane changes the physicochemical properties of the cell. This could effect for example the cell-electrode interaction. Therefore, we investigated the cellular surface characteristic by comparing ''Escherichia coli'' KRX wild type with ''Escherichia coli'' KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart> (Table 2 and Figure 5).</p> |
| - | *<p align="justify">The OprF strain shows an increasing affinity to hexadecane with increasing | + | *<p align="justify">The OprF strain shows an increasing affinity to hexadecane with increasing promoter strength in comparison to the wild type. Cells in which OprF was expressed from the T7 promoter (<bbpart>BBa_K1172502</bbpart>) showed the maximal hydrophobicity, which was three times higher than the affinity to hexadecane of the wild type.</p> |
| - | [[Image:IGEM_Bielefeld_Table5_Hexadecan.jpg|300px|thumb|left|<p align="justify"> '''Table 2: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#Hexadecan Assay | Hexadecane-Hydrophobicity-Assay]]. Comparison of | + | [[Image:IGEM_Bielefeld_Table5_Hexadecan.jpg|300px|thumb|left|<p align="justify"> '''Table 2: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#Hexadecan Assay | Hexadecane-Hydrophobicity-Assay]]. Comparison of hydrophobicity between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Affinity to hexadecane (hydrophobicity) with standard deviation and enhancement in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_Figure7_Results_Hexadecane.jpg|300px|thumb|center|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_Figure7_Results_Hexadecane.jpg|300px|thumb|center|<p align="justify"> '''Figure 5: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#Hexadecan Assay | Hexadecane-Hydrophobicity-Assay]]. Comparison of hydrophobicity between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Affinity to hexadecane (hydrophobicity) with standard deviation and enhancement in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | *<p align="justify">The enhanced hydrophobicity of OprF | + | *<p align="justify">The enhanced hydrophobicity of OprF expressing strains indicates a successful expression of the outer membrane porin in ''Escherichia coli''. Such an increased hydrophobicity on the outer membrane caused by the expression of OprF will lead to an increase in the cellular adhesion to the surface of the carbon anode and an enhancement of direct electron transfer from ''Escherichia coli'' to the electrode.</p> |
| - | ===Membrane | + | ===Membrane permeability assays=== |
====NPN uptake assay==== | ====NPN uptake assay==== | ||
| - | *<p align="justify">Besides testing the outer membrane hydrophobicity for physicochemical characterization of the ''E. coli'' surface, we measured the membrane permeability by [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay | NPN uptake assay]] for outer membrane morphology characterization (Helander and Mattila-Sandholm, 2000).</p> | + | *<p align="justify">Besides testing the outer membrane hydrophobicity for physicochemical characterization of the ''E. coli'' surface, we measured the membrane permeability by fluorescence-based [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay | NPN (N-Phenyl-1-naphthylamine) uptake assay]] for outer membrane morphology characterization (Helander and Mattila-Sandholm, 2000).</p> |
| - | *<p align="justify">NPN is a | + | *<p align="justify">NPN is a suitable chemical for measuring the membrane permeability of cells. An increasing NPN fluorescence intensity indicates an enhanced NPN uptake through the outer membrane and enhanced membrane permeability ([[Team:Bielefeld-Germany/Project/Porins#References|Loh ''et al''., 1984]]).</p> |
| - | *<p align="justify">Figure | + | *<p align="justify">Figure 6 shows a higher fluorescence emission and hence higher membrane permeability with increasing promoter strength for OprF strains in comparison to ''Escherichia coli'' KRX wild type.</p> |
| - | [[Image:IGEM_Bielefeld_Figure8_Results_NPN2.jpg|450px|thumb|left|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_Figure8_Results_NPN2.jpg|450px|thumb|left|<p align="justify"> '''Figure 6: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay | NPN-uptake-assay]]. Comparison of fluorescence emission between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Excitation at 355 nm with fluorescence emission scan from 320 to 390 nm wavelength and with standard deviation. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | *<p align="justify">''Escherichia coli'' with heterologous expression of the outer membrane porin OprF shows more efficient NPN | + | *<p align="justify">''Escherichia coli'' with heterologous expression of the outer membrane porin OprF shows more efficient NPN uptake than the ''Escherichia coli'' KRX wild type, suggesting an increased membrane permeability with increasing promoter strength. ''E. coli'' with ''oprF'' under T7 promoter control (<bbpart>BBa_K1172502</bbpart>) shows the maximal membrane permeability with 100% enhanced permeability in comparison to ''E. coli'' KRX wild type. The weak Anderson promoters seem unsuitable for heterologous expression of this gene.</p> |
| Line 152: | Line 161: | ||
| - | *<p align="justify">Another way of characterizing the outer cell membrane can be achieved | + | *<p align="justify">Another way of characterizing the outer cell membrane can be achieved with an fluorescence-based [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG assay]]. [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |This assay]] measures the membrane permeability by whole-cell lactase enzyme activity ([[Team:Bielefeld-Germany/Project/Porins#References|Zhou ''et al''., 2010]]).</p> |
| - | *<p align="justify">Electron shuttle-mediated electron transfer (EET) determines the transport efficiency of electron shuttles across the cell membrane. With [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay|NPN uptake assay]] we were able to quantify the transport efficiency of chemical molecules across the cell membrane. [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG assay]] ( | + | *<p align="justify">Electron shuttle-mediated electron transfer (EET) determines the transport efficiency of electron shuttles across the cell membrane. With the [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay|NPN uptake assay]] we were able to quantify the transport efficiency of chemical molecules across the cell membrane. In contrast, the [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG assay]] (whole-cell β-lactase enzyme activity assay) measures not only the diffusion of ONPG into the cell but also the release of its hydrolytic product across the cell membrane. With the [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG assay]] we are able to observe diffusion processes in and out of the cell membrane.</p> |
| - | *<p align="justify">Due the fact that heterologous expression of OprF in ''Escherichia coli'' | + | *<p align="justify">Due to the fact that heterologous expression of OprF in ''Escherichia coli'' should not have an impact on the expression of β-lactase, we can assume that the β-lactase activity of ''E. coli'' KRX wild type and ''E. coli'' KRX with OprF expression plasmid is identical. All in all, the [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG assay]] provides significantly more information than the [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay|NPN uptake assay]].</p> |
| - | [[Image:IGEM_Bielefeld_Figure9_Results_ONPG2.jpg|450px|thumb|left|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_Figure9_Results_ONPG2.jpg|450px|thumb|left|<p align="justify"> '''Figure 7: Results of the [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG-uptake-assay]]. Comparison of ONPG hydrolysis between ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Absorbance at 405 nm wavelength with standard deviation is shown. Two biological and at least three technical replicates were analyzed. '''</p>]] |
| - | *<p align="justify">The ONPG hydrolysis rate by β-lactase is much higher for '' | + | *<p align="justify">The ONPG hydrolysis rate by β-lactase is much higher for ''E. coli'' KRX containing OprF plasmids in contrast to ''E. coli'' KRX wild type. Whereas we could only observe a minimal hydrolysis activity in the wild type, ''E. coli'' KRX, carrying OprF expression plasmids, shows a much faster ONPG hydrolysis rate with increasing promoter strength and a maximal rate for'' E. coli'' KRX with T7 promoter (<bbpart>BBa_K1172502</bbpart>), which is 30 times higher than wild type hydrolysis rate.</p> |
| - | *<p align="justify">In summary we | + | *<p align="justify">In summary, we could prove that the heterologous expression of OprF from ''Pseudomonas fluorescens'' in ''Escherichia coli'' significantly improves the membrane permeability. The [[Team:Bielefeld-Germany/Labjournal/Molecular#NPN membrane permeability assay|NPN]] and [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG]] assay show comparable results. Outer membrane permeability of ''E. coli'' is enhanced with increasing promoter strength for OprF expression. The wild type shows only a weak uptake rate of chemical molecules (NPN) and no product release as quantified with [[Team:Bielefeld-Germany/Labjournal/Molecular#ONPG membrane permeability assay |ONPG assay]]. Therefore, the ''E. coli'' wild type is not well suitable for a usage in the MFC using mediators. In contrast, membrane optimized ''Escherichia coli'' with OprF shows increased diffusion across the cellular membrane, indicating a possible optimization of electron shuttle-mediated electron transfer (EET) to the anode and increasing current production.</p> |
| Line 169: | Line 178: | ||
| - | *<p align="justify">In addition to | + | *<p align="justify">In addition to the morphological and physicochemical characterization of the ''Escherichia coli'' outer membrane, we wanted to visualize the surface. The technique of choice for this is Atomic Force Microscopy (AFM). [[Team:Bielefeld-Germany/Labjournal/Molecular#Preparing samples for Atomic Force Microscopy (AFM) analysis of the OM of cells|After cell preparation]] we were able to get AFM pictures of the ''E. coli'' surface with the help of the working group of [http://www.physik.uni-bielefeld.de/biophysik Prof. Dr. Dario Anselmetti], with special help from [http://www.physik.uni-bielefeld.de/biophysik/mitarbeiter/walhorn.html Dr. Volker Walhorn]. Thank you very much for your help!</p> |
| - | [[Image:IGEM_Bielefeld_AFM_Preparation_WT.jpg|300px|thumb|left|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_AFM_Preparation_WT.jpg|300px|thumb|left|<p align="justify"> '''Figure 8: Light microscopy of AFM layer after [[Team:Bielefeld-Germany/Labjournal/Molecular#Preparing samples for Atomic Force Microscopy (AFM) analysis of the OM of cells|cell preparation]] of ''Escherichia coli'' KRX wild type. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_AFM_Preparation_OprF.jpg|300px|thumb|center|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_AFM_Preparation_OprF.jpg|300px|thumb|center|<p align="justify"> '''Figure 9: Light microscopy of AFM layer after [[Team:Bielefeld-Germany/Labjournal/Molecular#Preparing samples for Atomic Force Microscopy (AFM) analysis of the OM of cells|cell preparation]] of ''Escherichia coli'' carrying ''oprF'' under the control of T7 promoter (<bbpart>BBa_K1172502</bbpart>). Enhanced cell clustering, caused by higher hydrophobicity, can be observed. '''</p>]] |
| - | *<p align="justify">Microscopy of AFM glass layer after [[Team:Bielefeld-Germany/Labjournal/Molecular#Preparing samples for Atomic Force Microscopy (AFM) analysis of the OM of cells|cell preparation]] | + | *<p align="justify">Microscopy of the AFM glass layer after [[Team:Bielefeld-Germany/Labjournal/Molecular#Preparing samples for Atomic Force Microscopy (AFM) analysis of the OM of cells|cell preparation]], before AFM measurement, shows the different cell properties. Number of cells as well as magnification scale are the same. ''Escherichia coli'' KRX expressing OprF from a T7 promoter (<bbpart>BBa_K1172502</bbpart>) presents a clustering of cells, whereas the wild type forms a monolayer. Thus, the increased hydrophobicity of the cell surface is already visible even with an ordinary light microscope.</p> |
| Line 182: | Line 191: | ||
| - | [[Image:IGEM_Bielefeld_AFMscans_OprF.jpg|600px|thumb|left|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_AFMscans_OprF.jpg|600px|thumb|left|<p align="justify"> '''Figure 10: AFM images of the cell surface for ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX with heterologous expression of porin OprF (<bbpart>BBa_K1172502</bbpart>). Images are shown in Topography and Contrast mode. '''</p>]] |
| - | *<p align="justify"> | + | *<p align="justify">Based on the AFM images, ''Escherichia coli'' KRX with OprF and T7 promoter (<bbpart>BBa_K1172502</bbpart>) shows a slightly rougher cell surface morphology in contrast to ''Escherichia coli'' KRX wild type. The outer membrane porin OprF from ''Pseudomonas fluorescens'' usually forms trimer complexes on the membrane, which leads to enhanced roughness of the cell surface. ([[Team:Bielefeld-Germany/Project/Porins#References|Yong ''et al''., 2013]])</p> |
| - | *<p align="justify">The heterologous expression of porin OprF causes a slightly rougher membrane | + | *<p align="justify">The heterologous expression of the porin OprF causes a slightly rougher membrane, the morphology and thus the viability of'' Escherichia coli'' is preserved to the greatest possible extent.</p> |
| Line 192: | Line 201: | ||
| - | *<p align="justify">All results | + | *<p align="justify">All results so far indicated the potential for an increase in energy production. This assumption was confirmed by cultivation of'' Escherichia coli'' KRX with OprF (<bbpart>BBa_K1172502</bbpart>) in comparison to ''Escherichia coli'' KRX wild type in the Microbial Fuel Cell.</p> |
| - | *<p align="justify"> | + | *<p align="justify">Indeed, the extracellular electron transfer mediated by electron shuttles is improved in the OprF expressing strain resulting in an increased bioelectricity output (Figure 11 - 14).</p> |
| - | [[Image:IGEM_Bielefeld_Voltage2_OprF_Genetic.jpg|300px|thumb|left|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_Voltage2_OprF_Genetic.jpg|300px|thumb|left|<p align="justify"> '''Figure 11: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX with OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Voltage curve from ''Escherichia coli'' KRX wild type, ''Escherichia coli'' KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>), M9 medium with Methylene Blue as mediator and M9 medium without mediator is shown over time and over a resistance of 200 Ω. '''</p>]] |
| - | [[Image:IGEM_Bielefeld_ElectricCharge_OprF_Genetic.jpg|300px|thumb|center|<p align="justify"> '''Figure | + | [[Image:IGEM_Bielefeld_ElectricCharge_OprF_Genetic.jpg|300px|thumb|center|<p align="justify"> '''Figure 12: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Electric charge curve from ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator Methylene Blue and with measurement over a resistance of 200 Ω. '''</p>]] |
| + | [[Image:IGEM_Bielefeld_ElectricPowerOprF2.jpg|300px|thumb|left|<p align="justify"> '''Figure 13: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Electric power curve from ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator Methylene Blue and with measurement over a resistance of 200 Ω. '''</p>]] | ||
| + | [[Image:IGEM_Bielefeld2013_OprF_AverageElectricPower.jpg|300px|thumb|center|<p align="justify"> '''Figure 14: Microbial Fuel Cell results from cultivation of ''Escherichia coli'' KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to ''Escherichia coli'' KRX wild type. Average electric power bar chart from ''Escherichia coli'' KRX wild type and ''Escherichia coli'' KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator Methylene Blue and with measurement over a resistance of 200 Ω. '''</p>]] | ||
| - | *<p align="justify">''Escherichia coli'' KRX with OprF (<bbpart>BBa_K1172502</bbpart>) shows 108 % higher maximal voltage than ''Escherichia coli'' KRX | + | *<p align="justify">''Escherichia coli'' KRX with OprF (<bbpart>BBa_K1172502</bbpart>) shows 108 % higher maximal voltage than ''Escherichia coli'' KRX wild type with a maximum of 308 mV. Over the whole cultivation the voltage was improved by about 100 %. Tests using M9-medium with and without mediator Methylene Blue show no voltage, indicating that bioelectricity generation is solely due to the bacteria.</p> |
| - | *<p align="justify">The calculation of the electric charge confirms the described results. Electric charge is equivalent to the number of transported electrons and 111 % | + | *<p align="justify">The calculation of the electric charge confirms the described results. Electric charge is equivalent to the number of transported electrons and is enhanced by 111 % for ''Escherichia coli'' KRX with OprF (<bbpart>BBa_K1172502</bbpart>). The maximal electric charge of 7,2 C demonstrates that heterologous expression of outer membrane porin OprF enhances extracellular electron transfer dramatically. High membrane permeability is crucial for efficient mediator transport across the membrane and therefore for high bioelectricity generation.</p> |
| + | *<p align="justify">Finally, the calculated power curve shows 239 % enhanced electric power for ''Escherichia coli'' KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>) with a maximal electric energy transfer rate of 475 μW and a 300% enhanced average electric power.</p> | ||
| Line 207: | Line 219: | ||
| - | *<p align="justify">Cell membranes are protective layers which are crucial for physiology of the bacteria. In consideration of the Microbial Fuel Cell, membrane is also a barrier which decreases mediator and thus electron exchange between bacteria and anode. With heterologous expression of the outer membrane porin OprF from ''Pseudomonas fluorescens'' in ''Escherichia coli'' we | + | *<p align="justify">Cell membranes are protective layers, which are crucial for physiology of the bacteria. In consideration of the Microbial Fuel Cell, the cell membrane is also a barrier which decreases mediator and thus electron exchange between bacteria and anode. With heterologous expression of the outer membrane porin OprF from ''Pseudomonas fluorescens'' in ''Escherichia coli'' we were able to enhance membrane permeability. With this optimized cell membrane surface we generate an efficient electron shuttle-mediated EET with decreased limitation of EET by membrane barrier. In contrast to a perforation of the membrane with chemicals, cell viability is maintained with OprF expression ([[Team:Bielefeld-Germany/Project/Porins#References|Liu ''et al''., 2012]]). Beside improvement of EET, enhanced hydrophobicity shows optimized cell adhesion to the anode for biofilm formation and direct electron transfer.</p> |
| - | *<p align="justify">The heterologous expression of OprF is a | + | *<p align="justify">The heterologous expression of OprF is a genetic strategy to optimize electron shuttle-mediated electron transfer as well as electricity generation in Microbial Fuel Cells. The most suitable and efficient OprF device for ''Escherichia coli'' is a combination with the rhamnose inducible T7 promoter (<bbpart>BBa_K1172502</bbpart>).</p> |
| Line 237: | Line 249: | ||
| - | <div id=" | + | <div id="asdf"> |
| + | <html> | ||
| + | <div id="nav2" style="width:210px; padding-bottom:5px; padding-left:15px;"> | ||
| + | |||
| + | <div class="navbutton" id="home" style="float:left; padding-left:55px; margin-left:10px; padding-top:0px;"> | ||
| + | <a href="https://2013.igem.org/Team:Bielefeld-Germany" title="Jump to Frontpage"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f6/Bielefeld-Germany2013-ButtonHome.png" height="15px"> | ||
| + | </a> | ||
| + | </div> | ||
| + | <div class="navbutton" id="top" style="float:left; padding-left:10px; margin-left:10px; padding-top:0px;"> | ||
| + | <a href="#" title="Jump to top"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/a/ab/Bielefeld-Germany2013-Up_orange_new.png" height="15px"> | ||
| + | </a> | ||
| + | </div> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | <div id="rightcol" style="width:210px; height:100%; overflow-y:auto; box-shadow:0px 0px 2px 0px grey;" padding:0px 20px;> | ||
__TOC__ | __TOC__ | ||
| - | + | </div> | |
| + | |||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
Latest revision as of 23:40, 28 October 2013
Porins
Overview - Porin OprF

Figure 1: Schematic overview of the enhancement mechanism of electron shuttle-mediated electron transfer between bacteria and the anode of MFCs by the porin OprF. Oxidized mediators diffuse into the periplasmatic space where they accept electrons. In turn, reduced mediators diffuse through outer membrane porins and donate their electrons to the electrode.
The Microbial Fuel Cell (MFC) can be a future environmentally friendly biotechnological application for the production of electrical energy. As a future alternative energy source, the bioelectricity generation must become more efficient. A major limiting factor is the low permeability of the bacterial membrane, hindering transport of electron shuttles through the membrane and thereby restricting the electron shuttle-mediated extracellular electron transfer (EET) from bacteria to electrodes. This results in a reduced electrical power output of the MFC. Therefore, we heterologously expressed the porin protein OprF from Pseudomonas fluorescens in Escherichia coli. This leads to a much higher current output in comparison to its parental strain (E. coli KRX). This is most likely caused by improved electron shuttle-mediated extracellular electron transfer through dramatically increased membrane permeability. The heterologous expression of the outer membrane porin OprF from Pseudomonas fluorescens in Escherichia coli is a great genetic strategy to overcome limitations due to the membrane and to increase electricity generation by microorganisms.
Theory
The efficiency of extracellular electron transfer is a major limiting factor for electricity power output of MFCs. The electron shuttle-mediated EET is the most common EET pathway for microorganisms in a Microbial Fuel Cell such as Escherichia coli (Logan, 2009).
The cell membrane is a natural protective layer enabling proper physiology of bacteria, but it is also a barrier for substrate exchange, because an efficient electron shuttle-mediated EET requires diffusion of shuttle molecules across the cell membrane (Figure 1). However, the bacterial outer membrane is a low permeable barrier for the transport of electron shuttles across the cell membrane, which limits among other things the efficiency of electron transport and is responsible for the low power output of MFCs up to now. One strategy to improve the electron shuttle mediated EET is to enhance the permeability of the cell membrane. Evolutionary strategies, e.g. application of a continuous cell stress, are very time-consuming and unpredictable. The chemical treatment with permeabilizers can perforate the outer membrane but has negative impact on the viability and metabolism of the cells. (Liu et al., 2012)
We thus hypothesize that genetic engineering of a highly permeable cell membrane would be beneficial. Therefore, we introduce pore forming proteins in the outer membrane. These so called porins are common bacterial outer membrane proteins, which can form water-filled channels across the membrane of Gram-negative bacteria. They allow hydrophilic substances to diffuse across the outer membrane (Hancock and Brinkman, 2002).
E. coli expresses different porines, for example OmpF and OmpC. But these natural occurring porines are only permeable for molecules smaller than 600 Da, which decreases the range of usable mediators and the mediator transport kinetics (Yong et al., 2013). Thus, we thought about enhancing the amount and size of pores in the outer cell membrane with much larger porins than those of E. coli.
OprF is a major outer membrane protein in Pseudomonas species, which acts as a non-specific porin protein and adhesin. OprF represents one of the largest pore sizes on bacterial outer membranes, allowing diffusion of polysaccharides in a range of 2000 to 3000 Da. In contrast to OprF, the general porin channels of E. coli are only permeable to sugars with sizes smaller than 600 Da. (Yong et al., 2013)
In due consideration of all facts, heterologous expression of the porin protein OprF from Pseudomonas fluorescens in E. coli will improve the EET between bacteria and electrodes by increase of membrane permeability. A heterologous expression of large porins improves, besides mediator efficiency, the spectrum of usable mediators. Thus, alternative, environmentally friendly mediators such as NADH and riboflavin might become applicable instead of the artificial systems used up to now.
Genetic Approach
The oprF gene from Pseudomonas fluorescens was cloned and heterologously expressed in Escherichia coli KRX under the control of different promoters (Table 1).
Figure 2: pSB1C3 – <bbpart>BBa_K1172501</bbpart> OprF BioBrick (1298 bp) was examined by restriction analysis and sequencing.
Results
Overview
Upon expression of the outer membrane porin protein OprF, the morphological and physicochemical characteristics of the E. coli surface were analyzed. SDS-PAGE combined with MALDI-TOF MS/MS, different membrane permeability assays (NPN and ONPG), a hydrophobicity assay and Atomic Force Microscopy (AFM) characterize the OprF BioBrick <bbpart>BBa_K1172501</bbpart>.
SDS-PAGE and MALDI-TOF
OprF was detected in an SDS-PAGE with a broad overexpression band. Protein isolation of the outer membrane porin OprF by release of periplasmic protein fraction from E. coli via cold osmotic shock using Cell fractionating buffer 2.3 was successful.

Figure 3: SDS-PAGE with [http://www.thermoscientific.com/ecomm/servlet/productsdetail_11152___13576050_-1 Prestained Protein Ladder from Thermo Scientific] as marker. Comparison of protein expression between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart> and <bbpart>BBa_K1172507</bbpart> after periplasmic protein fractioning with Cell fractionating buffer 2.3. The band used for the MALDI-TOF MS/MS is marked with an arrow.
The SDS-PAGE shows a significantly higher protein concentration in extracts from E.coli expressing OprF from a T7 promoter (<bbpart>BBa_K1172502</bbpart>). This is most likely caused by the higher membrane permeability (shown with NPN and ONPG uptake assay), allowing an increased release of membrane proteins by 0.2 % SDS. Nevertheless, a strong overexpression band can be observed at the expected OprF size of about 36 kDa for <bbpart>BBa_K1172502</bbpart>, which is equated with a strong expression and overproduction of OprF.
Furthermore we were able to identify the overexpressed outer membrane porin (Figure. 3) with MALDI-TOF MS/MS.
Tryptic digest of the gel band thought to represent OprF and analysis with MALDI-TOF confirmed the outer membrane porin with a Mascot Score of 222 against the bacteria database.
Growth characteristics
In order to test the effect of the protein expression on the cell growth, growth was measured as increase in optical density and plotted against time.
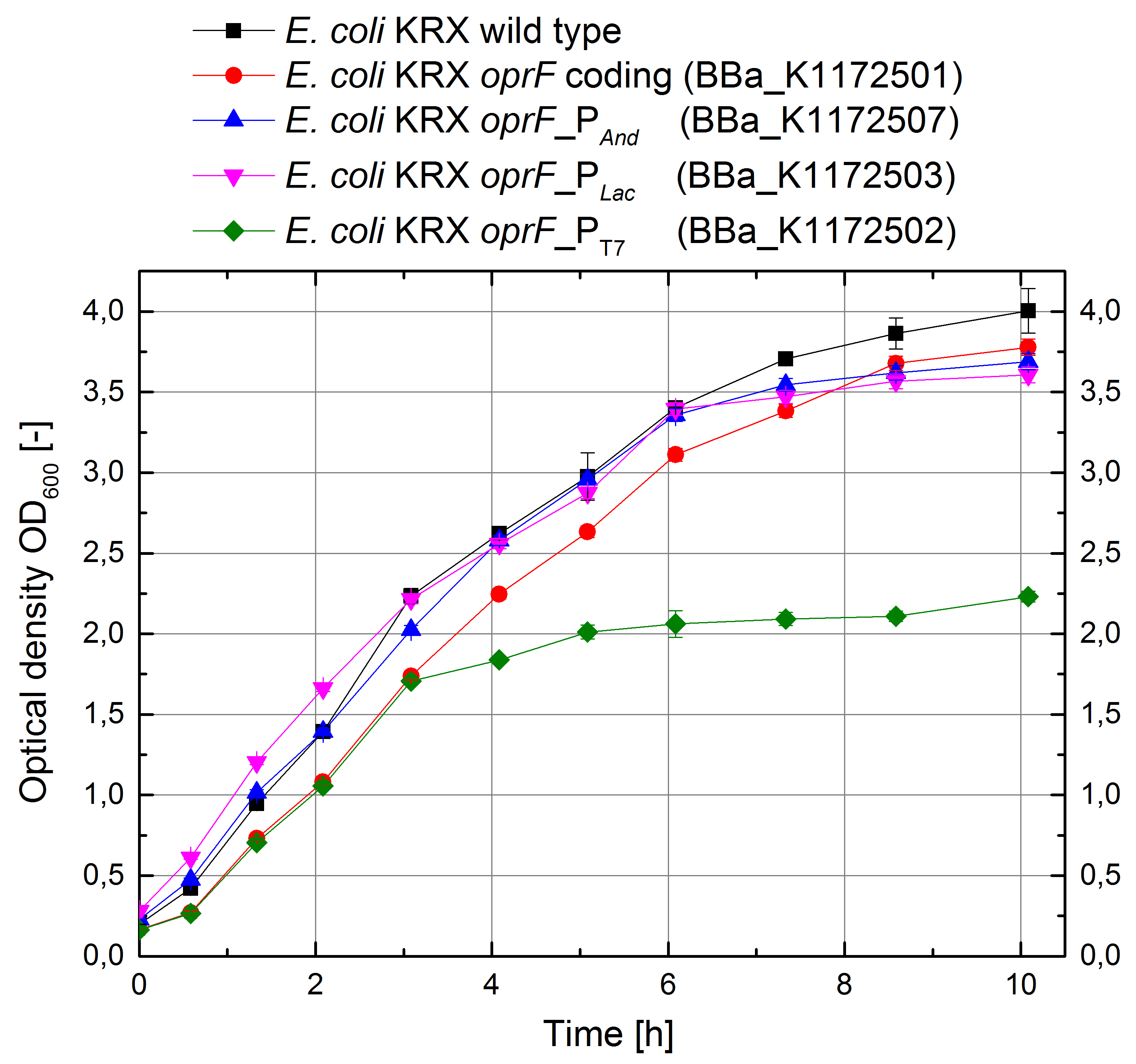
Figure 4: Growth curves for E. coli KRX with LB medium. Comparison between Escherichia coli KRX wild type and Escherichia coli KRX with oprF plasmids <bbpart>BBa_K1172501</bbpart>, <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart> and <bbpart>BBa_K1172507</bbpart>. oprF gene expression was induced for inducable promoters at OD600 = 1.0. All data are representing two biological and two technical replicates with standard deviation.
E. coli KRX wild type shows the best growth characteristics with a maximal optical density OD600 = 4.0. E. coli KRX with oprF coding plasmid shows a slightly lower growth due to the higher replication stress. E. coli KRX expressing oprF under control of strong Anderson and lac promoters are showing 10% decreased maximal cell density and E. coli KRX expressing oprF under control of T7 promoter shows a further decreased growth which was 45% reduced in comparison to E. coli KRX wild type. This lower growth is based on an increased expression of oprF on the cellular surface and therefore on higher metabolic pressure, which was confirmed with different assays, which can be found in the further characterization of the outer membrane protein OprF.
Hydrophobicity (Hexadecane) - Assay
The heterologous expression of the outer membrane porin OprF will enhance the hydrophobicity of cell membrane. The outward-facing side groups on each of the β-strands of the OprF monomer are hydrophobic. Therefore, heterologous expression should be detectable by an increase in hydrophobicity. The Hexadecane-Hydrophobicity-Assay is based on different affinities to hexadecane of dissimilar cell-types according to their cell surface. Hydrophobicity can be observed by specific changes in OD600 of the aqueous phase.
An increasing hydrophobicity of cell membrane changes the physicochemical properties of the cell. This could effect for example the cell-electrode interaction. Therefore, we investigated the cellular surface characteristic by comparing Escherichia coli KRX wild type with Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart> (Table 2 and Figure 5).
The OprF strain shows an increasing affinity to hexadecane with increasing promoter strength in comparison to the wild type. Cells in which OprF was expressed from the T7 promoter (<bbpart>BBa_K1172502</bbpart>) showed the maximal hydrophobicity, which was three times higher than the affinity to hexadecane of the wild type.
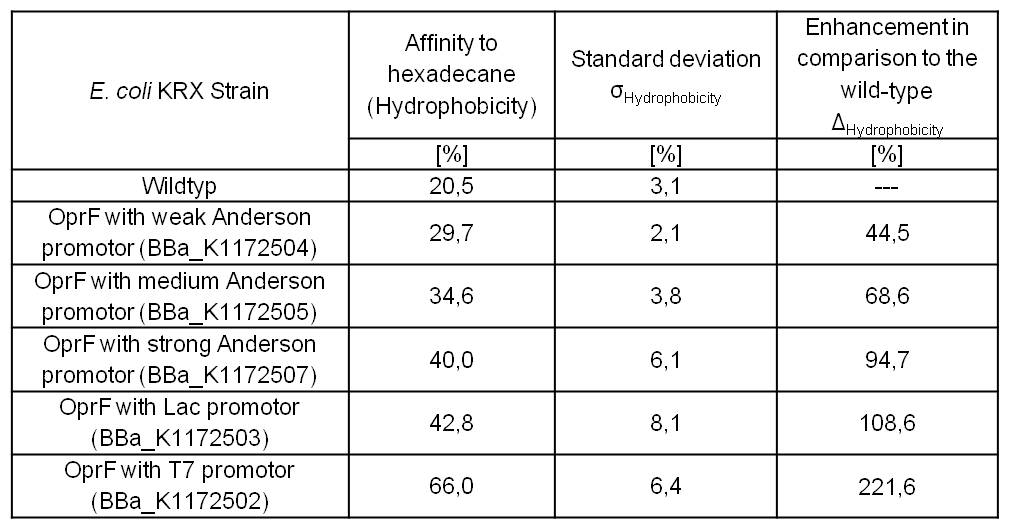
Table 2: Results of the Hexadecane-Hydrophobicity-Assay. Comparison of hydrophobicity between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Affinity to hexadecane (hydrophobicity) with standard deviation and enhancement in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.
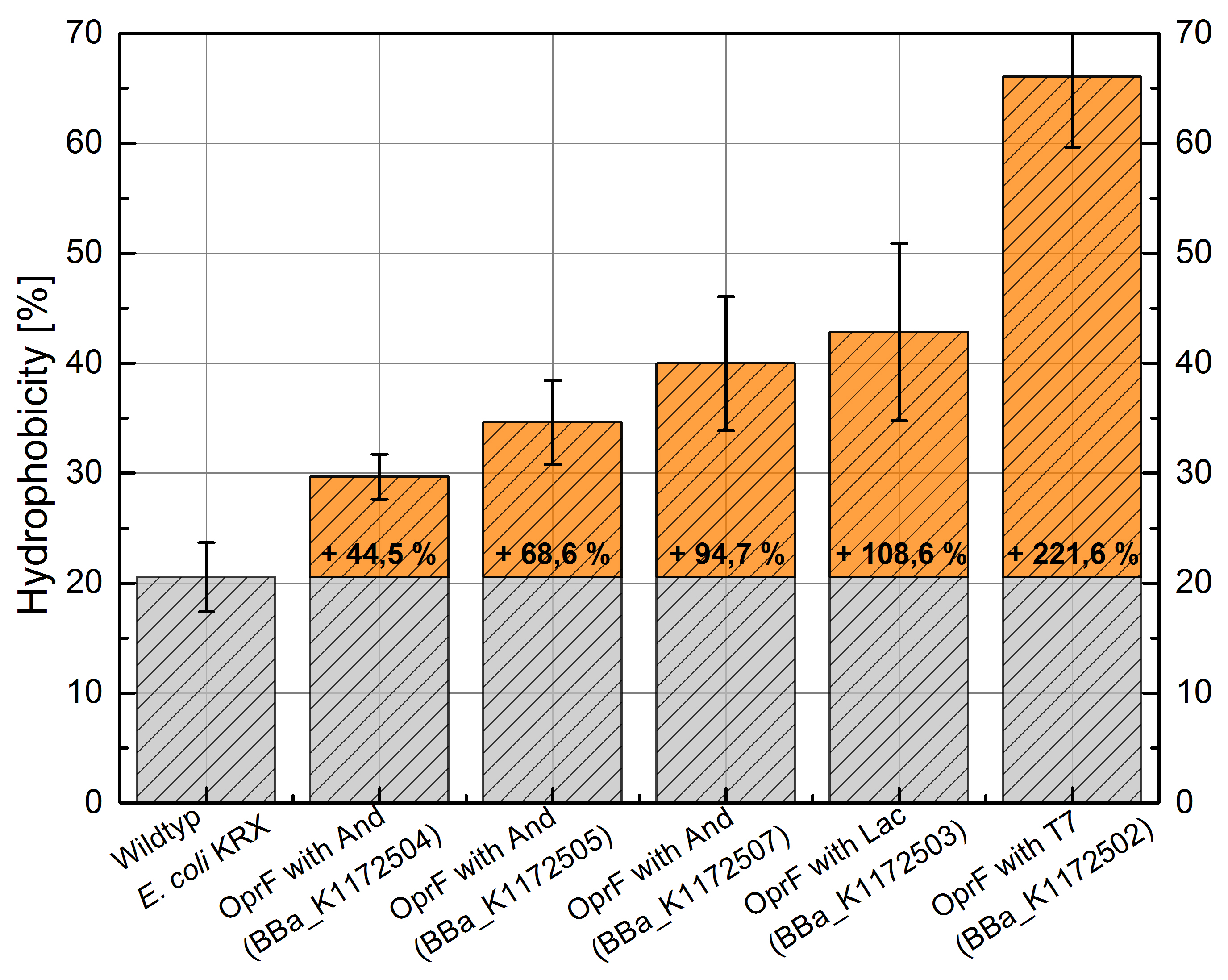
Figure 5: Results of the Hexadecane-Hydrophobicity-Assay. Comparison of hydrophobicity between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Affinity to hexadecane (hydrophobicity) with standard deviation and enhancement in comparison to the wild type is shown. Two biological and at least three technical replicates were analyzed.
The enhanced hydrophobicity of OprF expressing strains indicates a successful expression of the outer membrane porin in Escherichia coli. Such an increased hydrophobicity on the outer membrane caused by the expression of OprF will lead to an increase in the cellular adhesion to the surface of the carbon anode and an enhancement of direct electron transfer from Escherichia coli to the electrode.
Membrane permeability assays
NPN uptake assay
Besides testing the outer membrane hydrophobicity for physicochemical characterization of the E. coli surface, we measured the membrane permeability by fluorescence-based NPN (N-Phenyl-1-naphthylamine) uptake assay for outer membrane morphology characterization (Helander and Mattila-Sandholm, 2000).
NPN is a suitable chemical for measuring the membrane permeability of cells. An increasing NPN fluorescence intensity indicates an enhanced NPN uptake through the outer membrane and enhanced membrane permeability (Loh et al., 1984).
Figure 6 shows a higher fluorescence emission and hence higher membrane permeability with increasing promoter strength for OprF strains in comparison to Escherichia coli KRX wild type.
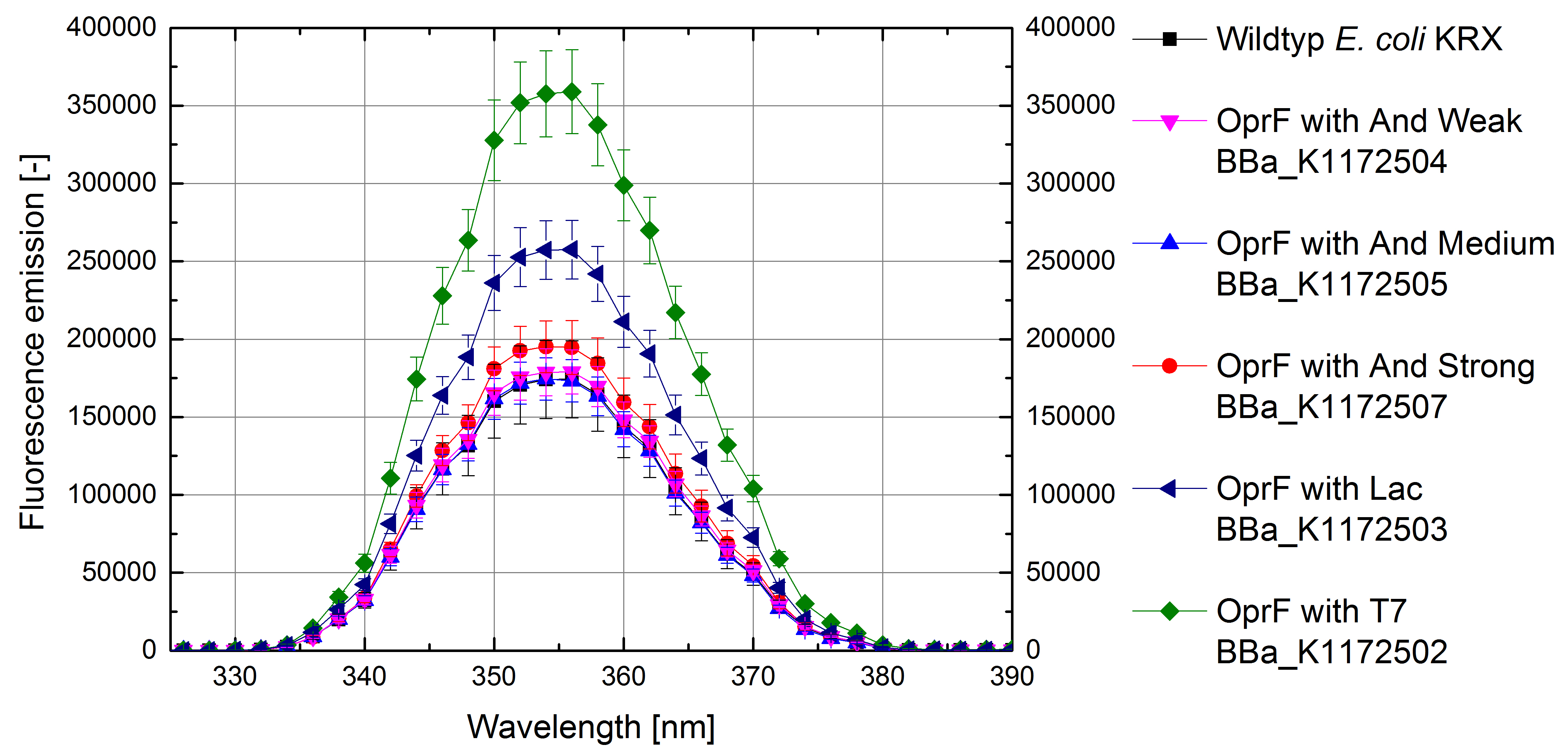
Figure 6: Results of the NPN-uptake-assay. Comparison of fluorescence emission between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Excitation at 355 nm with fluorescence emission scan from 320 to 390 nm wavelength and with standard deviation. Two biological and at least three technical replicates were analyzed.
Escherichia coli with heterologous expression of the outer membrane porin OprF shows more efficient NPN uptake than the Escherichia coli KRX wild type, suggesting an increased membrane permeability with increasing promoter strength. E. coli with oprF under T7 promoter control (<bbpart>BBa_K1172502</bbpart>) shows the maximal membrane permeability with 100% enhanced permeability in comparison to E. coli KRX wild type. The weak Anderson promoters seem unsuitable for heterologous expression of this gene.
ONPG assay
Another way of characterizing the outer cell membrane can be achieved with an fluorescence-based ONPG assay. This assay measures the membrane permeability by whole-cell lactase enzyme activity (Zhou et al., 2010).
Electron shuttle-mediated electron transfer (EET) determines the transport efficiency of electron shuttles across the cell membrane. With the NPN uptake assay we were able to quantify the transport efficiency of chemical molecules across the cell membrane. In contrast, the ONPG assay (whole-cell β-lactase enzyme activity assay) measures not only the diffusion of ONPG into the cell but also the release of its hydrolytic product across the cell membrane. With the ONPG assay we are able to observe diffusion processes in and out of the cell membrane.
Due to the fact that heterologous expression of OprF in Escherichia coli should not have an impact on the expression of β-lactase, we can assume that the β-lactase activity of E. coli KRX wild type and E. coli KRX with OprF expression plasmid is identical. All in all, the ONPG assay provides significantly more information than the NPN uptake assay.
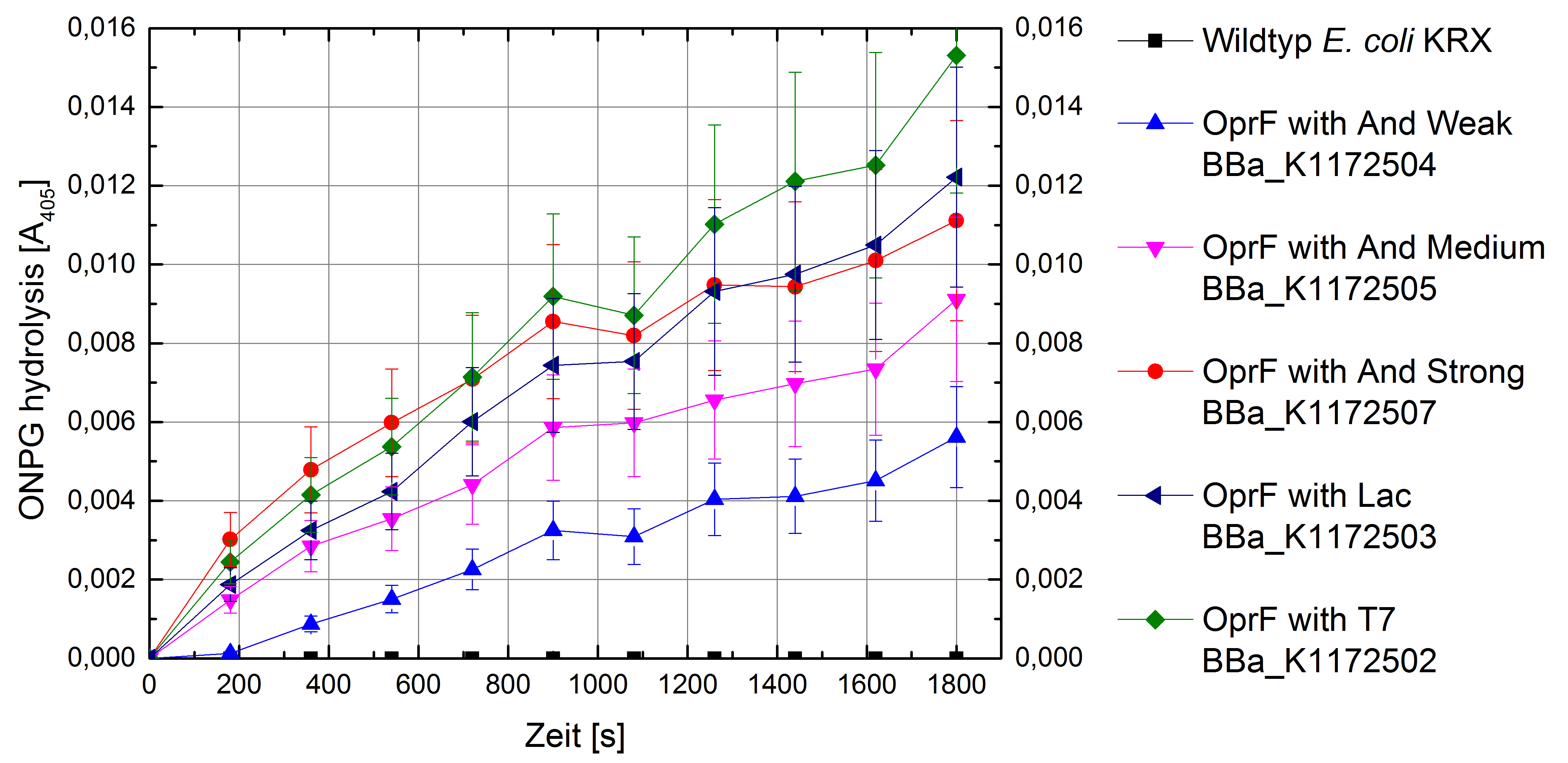
Figure 7: Results of the ONPG-uptake-assay. Comparison of ONPG hydrolysis between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Absorbance at 405 nm wavelength with standard deviation is shown. Two biological and at least three technical replicates were analyzed.
The ONPG hydrolysis rate by β-lactase is much higher for E. coli KRX containing OprF plasmids in contrast to E. coli KRX wild type. Whereas we could only observe a minimal hydrolysis activity in the wild type, E. coli KRX, carrying OprF expression plasmids, shows a much faster ONPG hydrolysis rate with increasing promoter strength and a maximal rate for E. coli KRX with T7 promoter (<bbpart>BBa_K1172502</bbpart>), which is 30 times higher than wild type hydrolysis rate.
In summary, we could prove that the heterologous expression of OprF from Pseudomonas fluorescens in Escherichia coli significantly improves the membrane permeability. The NPN and ONPG assay show comparable results. Outer membrane permeability of E. coli is enhanced with increasing promoter strength for OprF expression. The wild type shows only a weak uptake rate of chemical molecules (NPN) and no product release as quantified with ONPG assay. Therefore, the E. coli wild type is not well suitable for a usage in the MFC using mediators. In contrast, membrane optimized Escherichia coli with OprF shows increased diffusion across the cellular membrane, indicating a possible optimization of electron shuttle-mediated electron transfer (EET) to the anode and increasing current production.
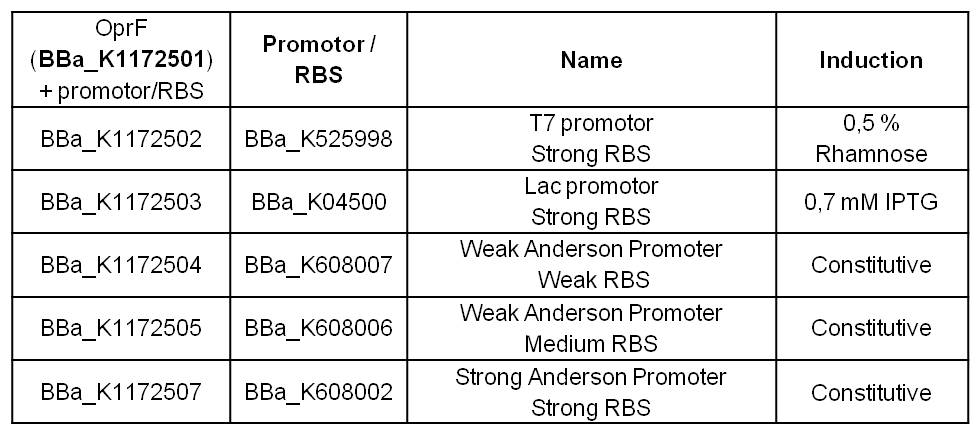
Atomic Force Microscopy (AFM)
In addition to the morphological and physicochemical characterization of the Escherichia coli outer membrane, we wanted to visualize the surface. The technique of choice for this is Atomic Force Microscopy (AFM). After cell preparation we were able to get AFM pictures of the E. coli surface with the help of the working group of [http://www.physik.uni-bielefeld.de/biophysik Prof. Dr. Dario Anselmetti], with special help from [http://www.physik.uni-bielefeld.de/biophysik/mitarbeiter/walhorn.html Dr. Volker Walhorn]. Thank you very much for your help!
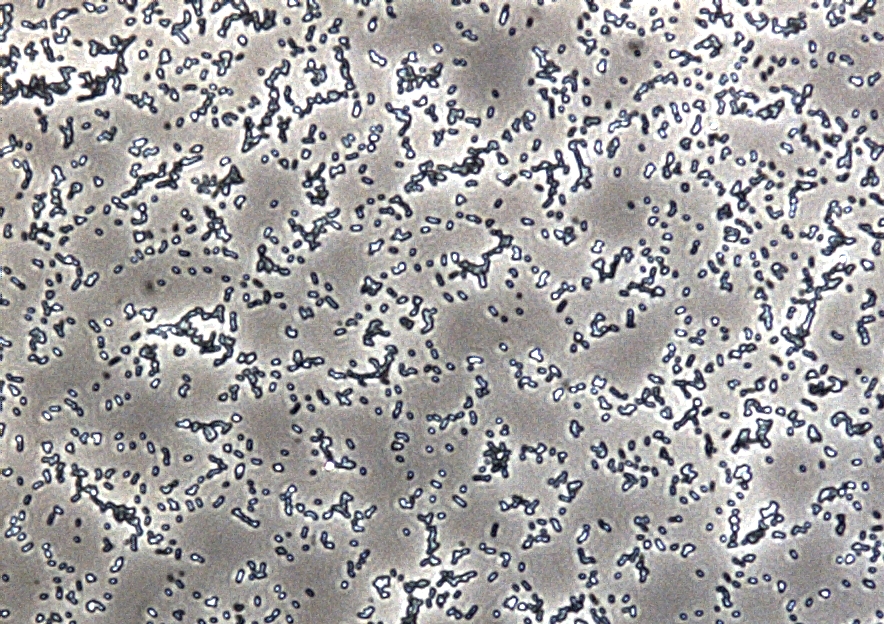
Figure 8: Light microscopy of AFM layer after cell preparation of Escherichia coli KRX wild type.
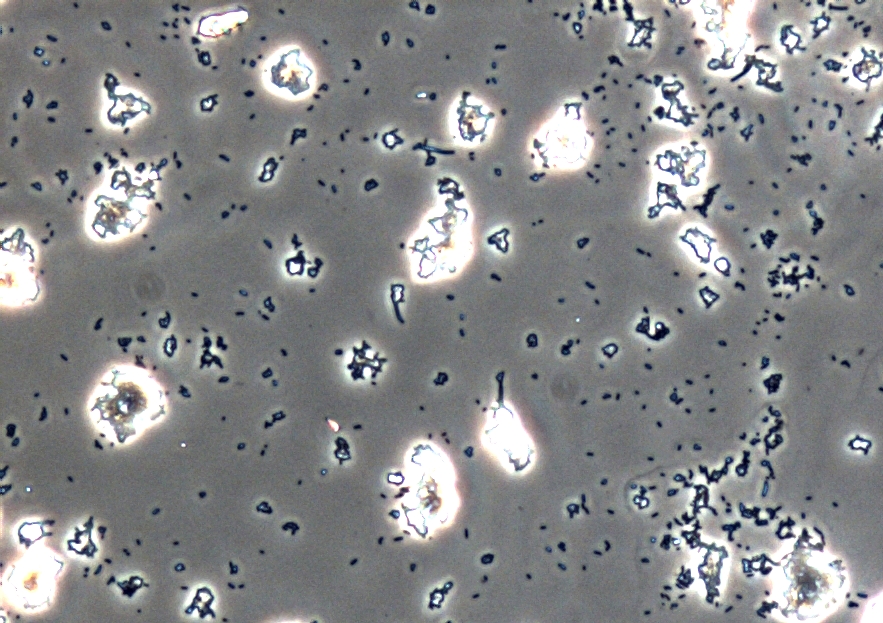
Figure 9: Light microscopy of AFM layer after cell preparation of Escherichia coli carrying oprF under the control of T7 promoter (<bbpart>BBa_K1172502</bbpart>). Enhanced cell clustering, caused by higher hydrophobicity, can be observed.
Microscopy of the AFM glass layer after cell preparation, before AFM measurement, shows the different cell properties. Number of cells as well as magnification scale are the same. Escherichia coli KRX expressing OprF from a T7 promoter (<bbpart>BBa_K1172502</bbpart>) presents a clustering of cells, whereas the wild type forms a monolayer. Thus, the increased hydrophobicity of the cell surface is already visible even with an ordinary light microscope.
AFM was carried out using the [http://www.bruker.com/de/products/surface-analysis/atomic-force-microscopy/multimode-8/overview.html MultiMode® 8 AFM from Bruker]. The measurements were performed on air with ‘Tapping Mode’ and in water with ‘Peak Force Mode’.
Based on the AFM images, Escherichia coli KRX with OprF and T7 promoter (<bbpart>BBa_K1172502</bbpart>) shows a slightly rougher cell surface morphology in contrast to Escherichia coli KRX wild type. The outer membrane porin OprF from Pseudomonas fluorescens usually forms trimer complexes on the membrane, which leads to enhanced roughness of the cell surface. (Yong et al., 2013)
The heterologous expression of the porin OprF causes a slightly rougher membrane, the morphology and thus the viability of Escherichia coli is preserved to the greatest possible extent.
Microbial Fuel Cell Measurement
All results so far indicated the potential for an increase in energy production. This assumption was confirmed by cultivation of Escherichia coli KRX with OprF (<bbpart>BBa_K1172502</bbpart>) in comparison to Escherichia coli KRX wild type in the Microbial Fuel Cell.
Indeed, the extracellular electron transfer mediated by electron shuttles is improved in the OprF expressing strain resulting in an increased bioelectricity output (Figure 11 - 14).
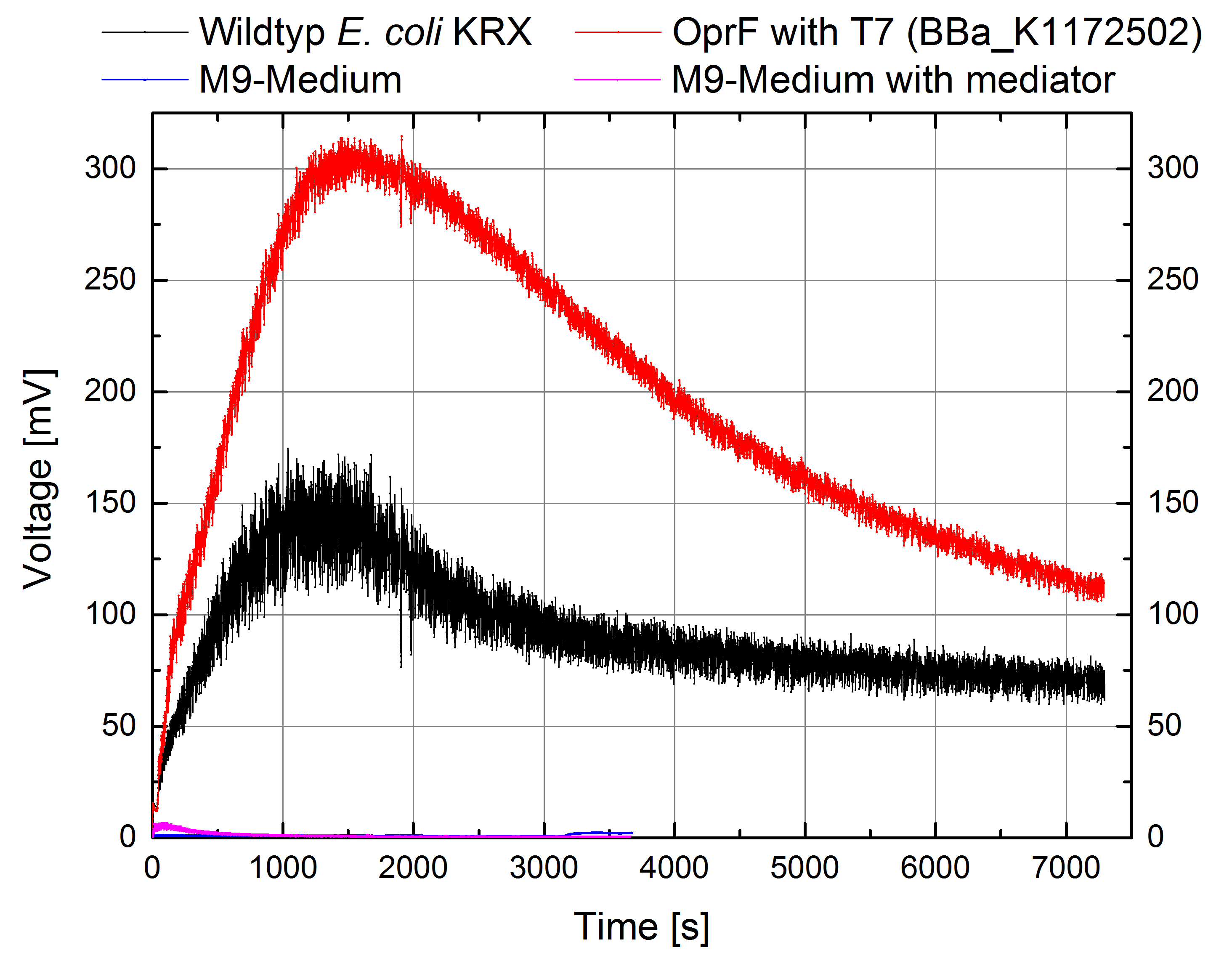
Figure 11: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type. Voltage curve from Escherichia coli KRX wild type, Escherichia coli KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>), M9 medium with Methylene Blue as mediator and M9 medium without mediator is shown over time and over a resistance of 200 Ω.
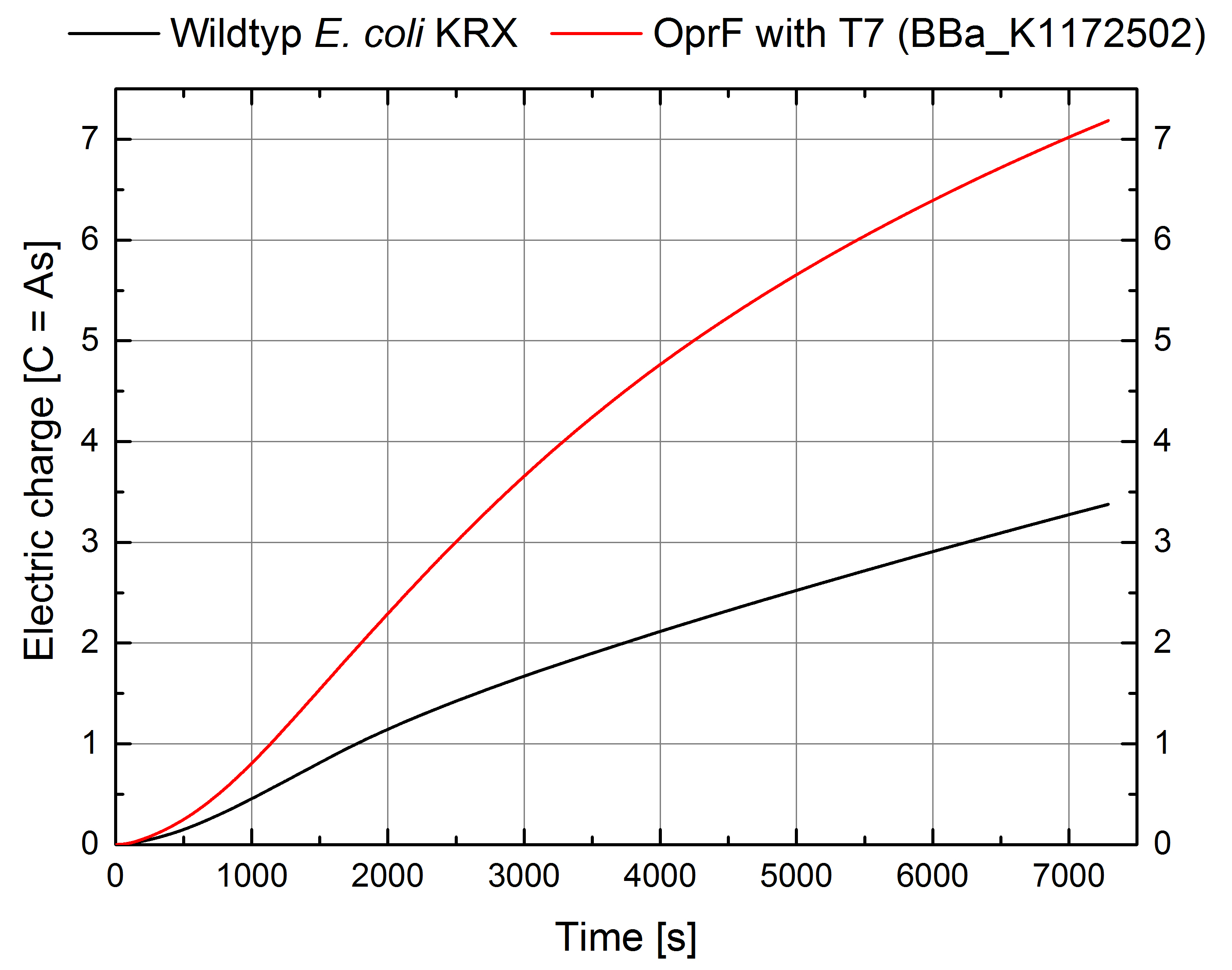
Figure 12: Microbial Fuel Cell results from cultivation of Escherichia coli KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type. Electric charge curve from Escherichia coli KRX wild type and Escherichia coli KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator Methylene Blue and with measurement over a resistance of 200 Ω.

Figure 13: Microbial Fuel Cell results from cultivation of Escherichia coli KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type. Electric power curve from Escherichia coli KRX wild type and Escherichia coli KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator Methylene Blue and with measurement over a resistance of 200 Ω.
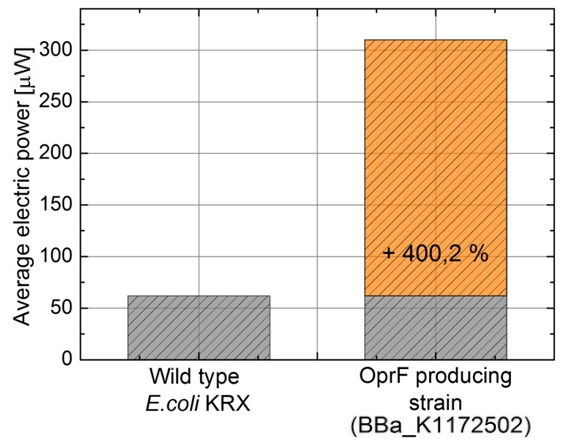
Figure 14: Microbial Fuel Cell results from cultivation of Escherichia coli KRX expressing OprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type. Average electric power bar chart from Escherichia coli KRX wild type and Escherichia coli KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator Methylene Blue and with measurement over a resistance of 200 Ω.
Escherichia coli KRX with OprF (<bbpart>BBa_K1172502</bbpart>) shows 108 % higher maximal voltage than Escherichia coli KRX wild type with a maximum of 308 mV. Over the whole cultivation the voltage was improved by about 100 %. Tests using M9-medium with and without mediator Methylene Blue show no voltage, indicating that bioelectricity generation is solely due to the bacteria.
The calculation of the electric charge confirms the described results. Electric charge is equivalent to the number of transported electrons and is enhanced by 111 % for Escherichia coli KRX with OprF (<bbpart>BBa_K1172502</bbpart>). The maximal electric charge of 7,2 C demonstrates that heterologous expression of outer membrane porin OprF enhances extracellular electron transfer dramatically. High membrane permeability is crucial for efficient mediator transport across the membrane and therefore for high bioelectricity generation.
Finally, the calculated power curve shows 239 % enhanced electric power for Escherichia coli KRX carrying OprF (<bbpart>BBa_K1172502</bbpart>) with a maximal electric energy transfer rate of 475 μW and a 300% enhanced average electric power.
Conclusion
Cell membranes are protective layers, which are crucial for physiology of the bacteria. In consideration of the Microbial Fuel Cell, the cell membrane is also a barrier which decreases mediator and thus electron exchange between bacteria and anode. With heterologous expression of the outer membrane porin OprF from Pseudomonas fluorescens in Escherichia coli we were able to enhance membrane permeability. With this optimized cell membrane surface we generate an efficient electron shuttle-mediated EET with decreased limitation of EET by membrane barrier. In contrast to a perforation of the membrane with chemicals, cell viability is maintained with OprF expression (Liu et al., 2012). Beside improvement of EET, enhanced hydrophobicity shows optimized cell adhesion to the anode for biofilm formation and direct electron transfer.
The heterologous expression of OprF is a genetic strategy to optimize electron shuttle-mediated electron transfer as well as electricity generation in Microbial Fuel Cells. The most suitable and efficient OprF device for Escherichia coli is a combination with the rhamnose inducible T7 promoter (<bbpart>BBa_K1172502</bbpart>).
References
Hancock REW, Brinkman FSL (2002) Function of Pseudomonas porins in uptake and efflux. [http://www.annualreviews.org/doi/abs/10.1146/annurev.micro.56.012302.160310| Annu Rev Microbiol (56):17–38].
Helander IM, Mattila-Sandholm T (2000) Permeability barrier of the gramnegative bacterial outer membrane with special reference to nisin. [http://www.sciencedirect.com/science/article/pii/S016816050000307X|Int J Food Microbiol 60: 153–161].
Liu J, Qiao Y, Lu ZS, Song H, Li CM (2012) Enhance electron transfer and performance of microbial fuel cells by perforating the cell membrane. [http://www.sciencedirect.com/science/article/pii/S1388248111004723|Electrochem Commun 15: 50–53].
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. [http://www.nature.com/nrmicro/journal/v7/n5/full/nrmicro2113.html| Nat Rev Microbiol (7): 375–381].
Loh B, Grant C, Hancock REW (1984) Use of the fluorescent-probe 1-Nphenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer-membrane of Pseudomonas aeruginosa. [http://aac.asm.org/content/26/4/546.short|Antimicrob Agents Chemother 26: 546–551].
Yong YC, Yu YY, Yang Y, Liu J, Wang JY, Song H (2013) Enhancement of Extracellular Electron Transfer and Bioelectricity Output by Synthetic Porin. [http://onlinelibrary.wiley.com/doi/10.1002/bit.24732/full|Biotechnology and Bioengineering 110 (2): 408-416].
Zhou ZX, Wei DF, Guan Y, Zheng AN, Zhong JJ (2010) Damage of Escherichia coli membrane by bactericidal agent polyhexamethylene guanidine hydrochloride: Micrographic evidences. [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2009.04482.x/full|J Appl Microbiol 108: 898–907].
 "
"