Team:Warsaw/Cytotoxicity
From 2013.igem.org
(→Overview) |
|||
| (10 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
The neurotoxic effects of acrylamide have been widely known for many years. Local symptoms of acrylamide poisoning such as mucous membrane and skin irritation have been formerly reported. There also exists a systemic effect characterized by fatigue, sleepiness and memory difficulties; in extreme cases also hallucination, disorientation and confusion were observed. | The neurotoxic effects of acrylamide have been widely known for many years. Local symptoms of acrylamide poisoning such as mucous membrane and skin irritation have been formerly reported. There also exists a systemic effect characterized by fatigue, sleepiness and memory difficulties; in extreme cases also hallucination, disorientation and confusion were observed. | ||
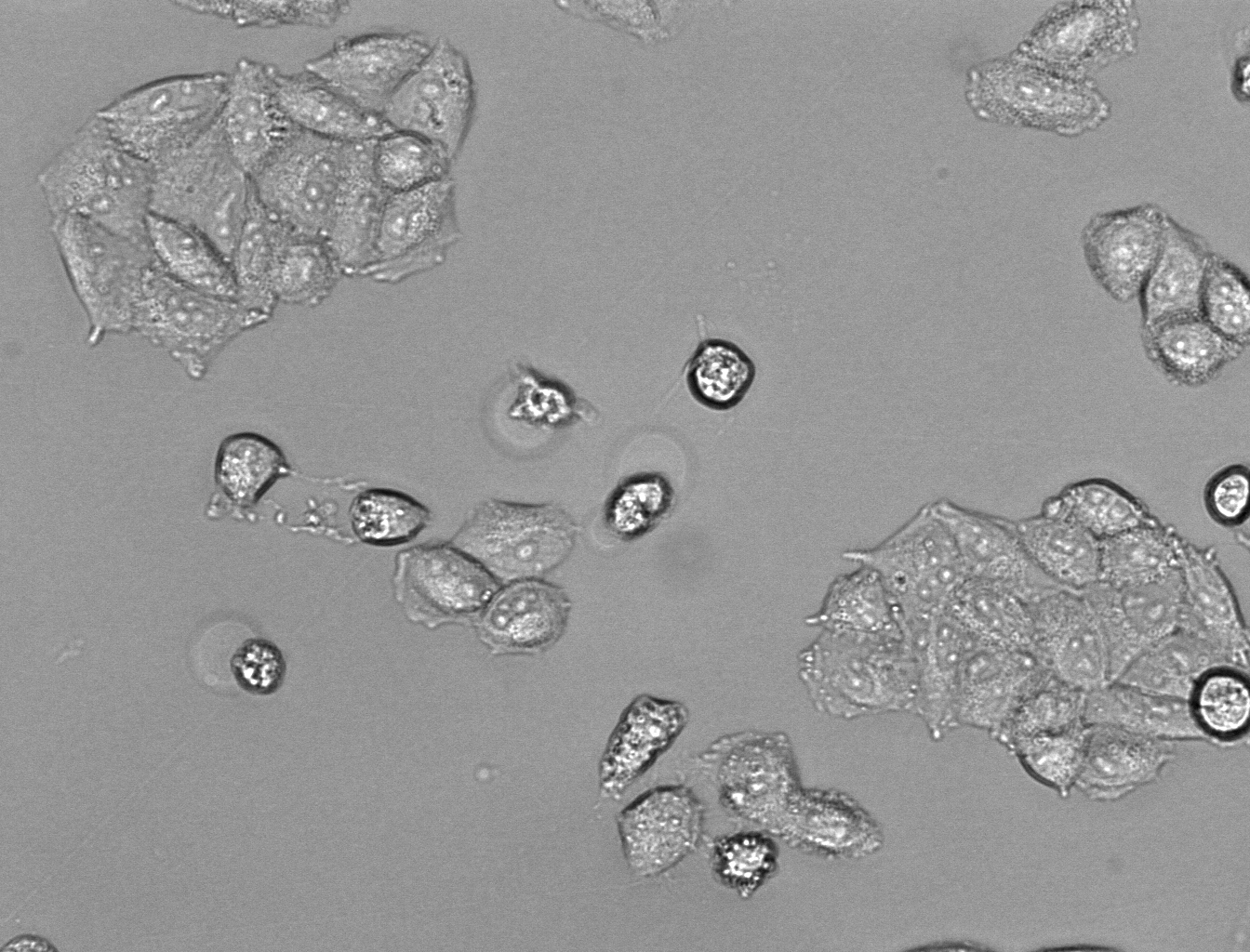
| - | [[File:Hela_24_4mM_2.png| | + | [[File:Hela_24_4mM_2.png|255px|thumb|left|alt=A|HeLa cells after 24h incubation with 4mM acrylamide.]] |
| + | |||
While acrylamide was perceived as a severe neurotoxin, there had long not been enough studies on its possible carcinogenicity. Yet, the recent research have shown that acrylamide may be engaged in processes. | While acrylamide was perceived as a severe neurotoxin, there had long not been enough studies on its possible carcinogenicity. Yet, the recent research have shown that acrylamide may be engaged in processes. | ||
| Line 10: | Line 11: | ||
Possible ways of acrylamide absorption are as follows: inhalation, ingestion, and through the skin and mucous membranes. Basing on a report of WHO from 2002 average daily intake for the general population ranges from 0.3 to 0.8 micrograms of acrylamide per kilogram of body mass. | Possible ways of acrylamide absorption are as follows: inhalation, ingestion, and through the skin and mucous membranes. Basing on a report of WHO from 2002 average daily intake for the general population ranges from 0.3 to 0.8 micrograms of acrylamide per kilogram of body mass. | ||
| + | |||
Increased consumption of deep-fried food and, subsequently, increased intake of acrylamide, may have serious health consequences. Recent studies have revealed an association between consumption of deep-fried foods and increased prostate cancer risk. What is also suggested, is that there is positive correlation between acrylamide intake and increased risk of endometrial and ovarian cancer. | Increased consumption of deep-fried food and, subsequently, increased intake of acrylamide, may have serious health consequences. Recent studies have revealed an association between consumption of deep-fried foods and increased prostate cancer risk. What is also suggested, is that there is positive correlation between acrylamide intake and increased risk of endometrial and ovarian cancer. | ||
| + | |||
| + | Considering the alarming effects of acrylamide exposure, we have decided to include in our project a cytotoxicity study. Our aim is to show how acrylamide may affect various cell lines originating from different tissues. In our experiments we are working on HeLa, 293 and 143B cell lines representing cervical cancer, human embryonic kidney and bone osteosarcoma cells respectively. | ||
[[File:Alamar1.JPG|250 px|thumb|right|alt=A|Probes for AlamarBlue.]] | [[File:Alamar1.JPG|250 px|thumb|right|alt=A|Probes for AlamarBlue.]] | ||
| - | |||
| Line 21: | Line 24: | ||
To indicate cell viability, we have decided to implement the Alamar Blue assay. It bases on the ability of living cells to convert nonfluorescent resazurin to the highly fluorescent molecule - resorufin. While entering the cells, resazurin is being reduced to resorufin and the bright, red fluorescence is observed. Viable cells are continuing to convert resazurin to resorufin, so the fluorescence intensity increases proportionally, which can be use to measure viability of the cells. | To indicate cell viability, we have decided to implement the Alamar Blue assay. It bases on the ability of living cells to convert nonfluorescent resazurin to the highly fluorescent molecule - resorufin. While entering the cells, resazurin is being reduced to resorufin and the bright, red fluorescence is observed. Viable cells are continuing to convert resazurin to resorufin, so the fluorescence intensity increases proportionally, which can be use to measure viability of the cells. | ||
| + | |||
[[File:Alamar2.JPG|190px|thumb|left|alt=A|Probes for Flow Cytometry]] | [[File:Alamar2.JPG|190px|thumb|left|alt=A|Probes for Flow Cytometry]] | ||
| Line 28: | Line 32: | ||
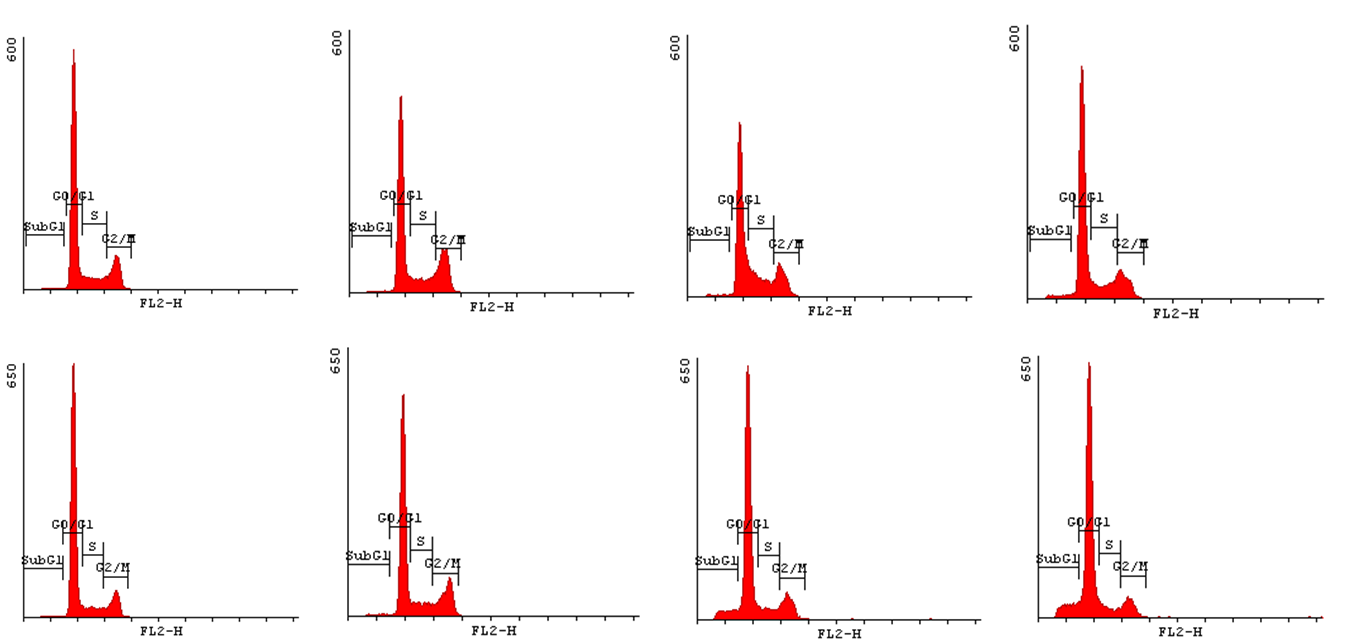
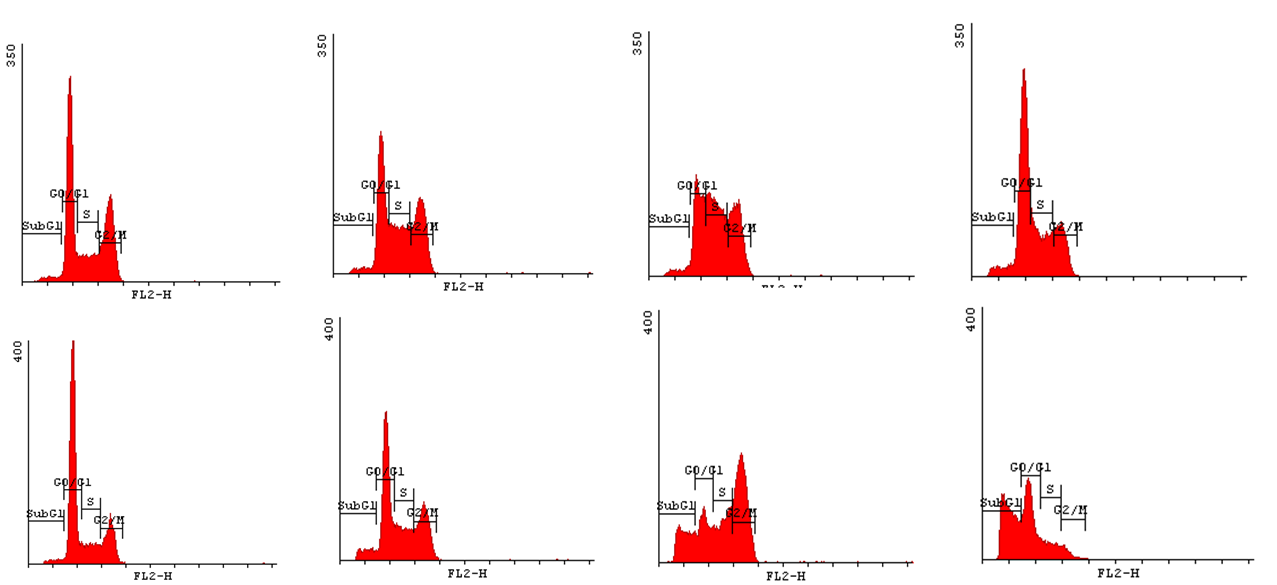
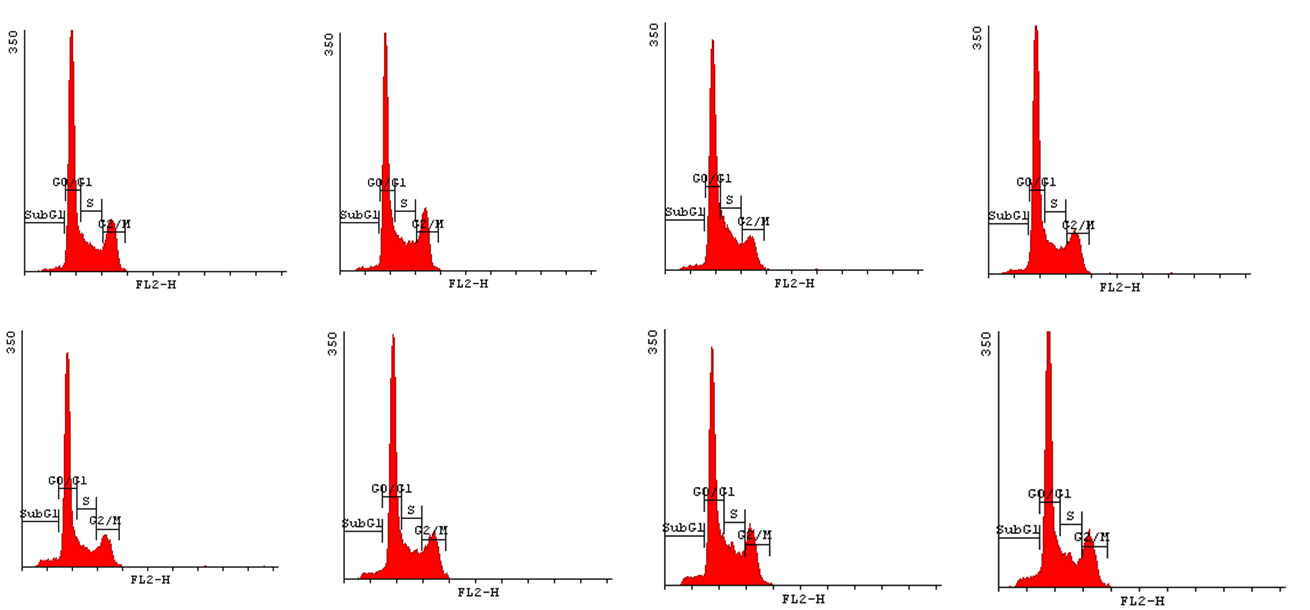
In order to state how acrylamide may influence the progression of cell cycle we have decided to implement Cell Cycle Assay. This method employs flow cytometry to mesure number of cells in a given phase of the cell cycle. Permeabilization of cells with cold ethanol and addition of propidium iodide (PI) prior to analysis, allows us to measure the fluorescence. PI binds to DNA quantitatively so the fluorescence intensity of the stained cells is correlated with the amount of their DNA. Thus, basing on the quantity of DNA we are enabled to assign cells to different cell cycle phases. | In order to state how acrylamide may influence the progression of cell cycle we have decided to implement Cell Cycle Assay. This method employs flow cytometry to mesure number of cells in a given phase of the cell cycle. Permeabilization of cells with cold ethanol and addition of propidium iodide (PI) prior to analysis, allows us to measure the fluorescence. PI binds to DNA quantitatively so the fluorescence intensity of the stained cells is correlated with the amount of their DNA. Thus, basing on the quantity of DNA we are enabled to assign cells to different cell cycle phases. | ||
| - | We expect to observe the cytotoxic effects in higher acrylamide concentrations i.e. 3-5 mM. In lower concentrations <1 mM may occur the elimination of the toxicant, whose effects are to be examined. | + | We expect to observe the cytotoxic effects in higher acrylamide concentrations i.e. 3-5 mM. In lower concentrations <1 mM may occur the elimination of the toxicant, whose effects are to be examined. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Results== | ==Results== | ||
| Line 53: | Line 50: | ||
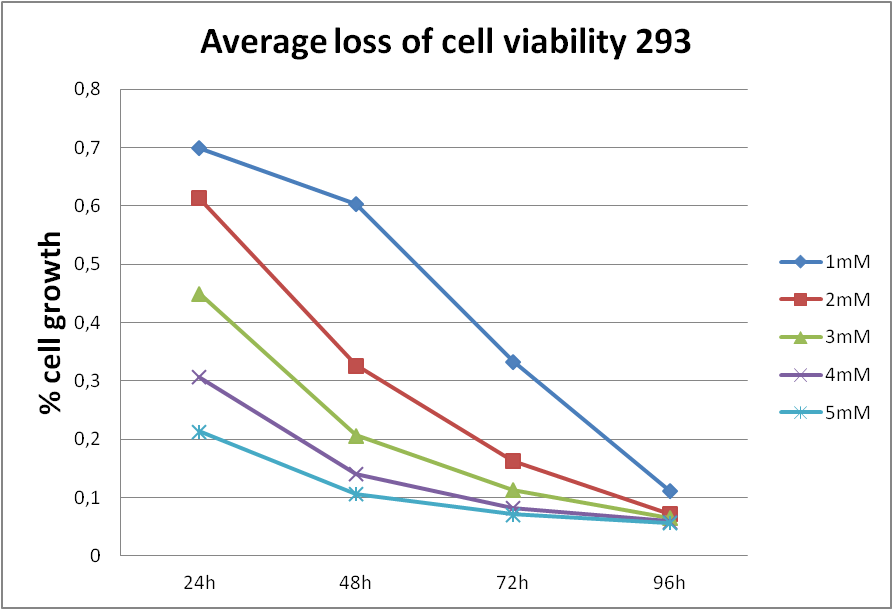
The outcome of AlamarBlue assay showed the existence of strong, negative correlation between acrylamide exposure and cell viability (Figure 1.). So the longer exposure time and the higher concentration of acrylamide, the weaker the cell viability. | The outcome of AlamarBlue assay showed the existence of strong, negative correlation between acrylamide exposure and cell viability (Figure 1.). So the longer exposure time and the higher concentration of acrylamide, the weaker the cell viability. | ||
| - | [[File:avHEK2.png|550px|frameless|border|alt=A|Figure 1. Influence of acrylamide on 293 cells viability. Cells were treated with various concentration of acrylamide and the viability was measured after indicated period of time.|]] | + | [[File:avHEK2.png|550px|center|frameless|border|alt=A|Figure 1. Influence of acrylamide on 293 cells viability. Cells were treated with various concentration of acrylamide and the viability was measured after indicated period of time.|]] |
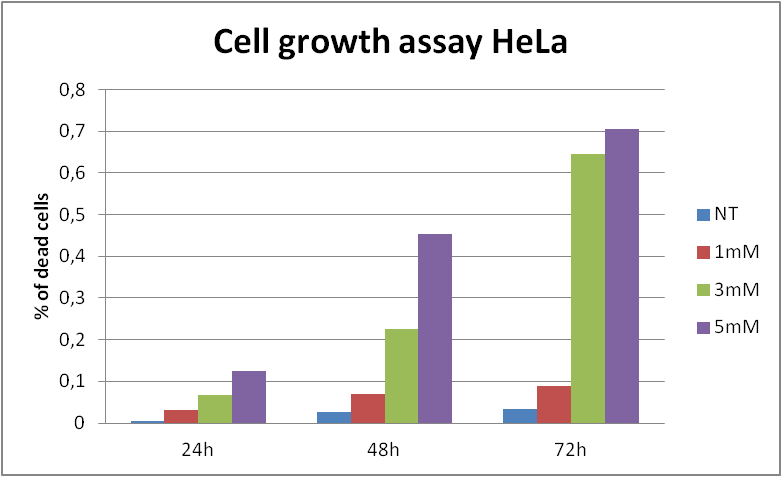
In order to state whether the loss of cell viability, that we have observed, was caused by decrease in cell division (the cytostatic effect of acrylamide) or by cell death (the cytotoxic effect of acrylamide) we have implemented Cell growth assays. To conduct the assays we have seeded cells from different cell lines (HeLa, 293 and 143B) in 6-wells plates adding proper concentration of acrylamide to each well and counted the amount of cells in counting chamber after trypan blue staining, for 24, 48 and 72 hours incubation. This approach indicated that acrylamide treatment affects cell proliferation and induces cell death. | In order to state whether the loss of cell viability, that we have observed, was caused by decrease in cell division (the cytostatic effect of acrylamide) or by cell death (the cytotoxic effect of acrylamide) we have implemented Cell growth assays. To conduct the assays we have seeded cells from different cell lines (HeLa, 293 and 143B) in 6-wells plates adding proper concentration of acrylamide to each well and counted the amount of cells in counting chamber after trypan blue staining, for 24, 48 and 72 hours incubation. This approach indicated that acrylamide treatment affects cell proliferation and induces cell death. | ||
| - | [[File:CG2HeLa.png|frameless|border|caption|850px]] | + | [[File:CG2HeLa.png|center|frameless|border|caption|850px]] |
| - | [[File:CGA-HeLa.png|frameless|border|caption|550px]] | + | [[File:CGA-HeLa.png|center|frameless|border|caption|550px]] |
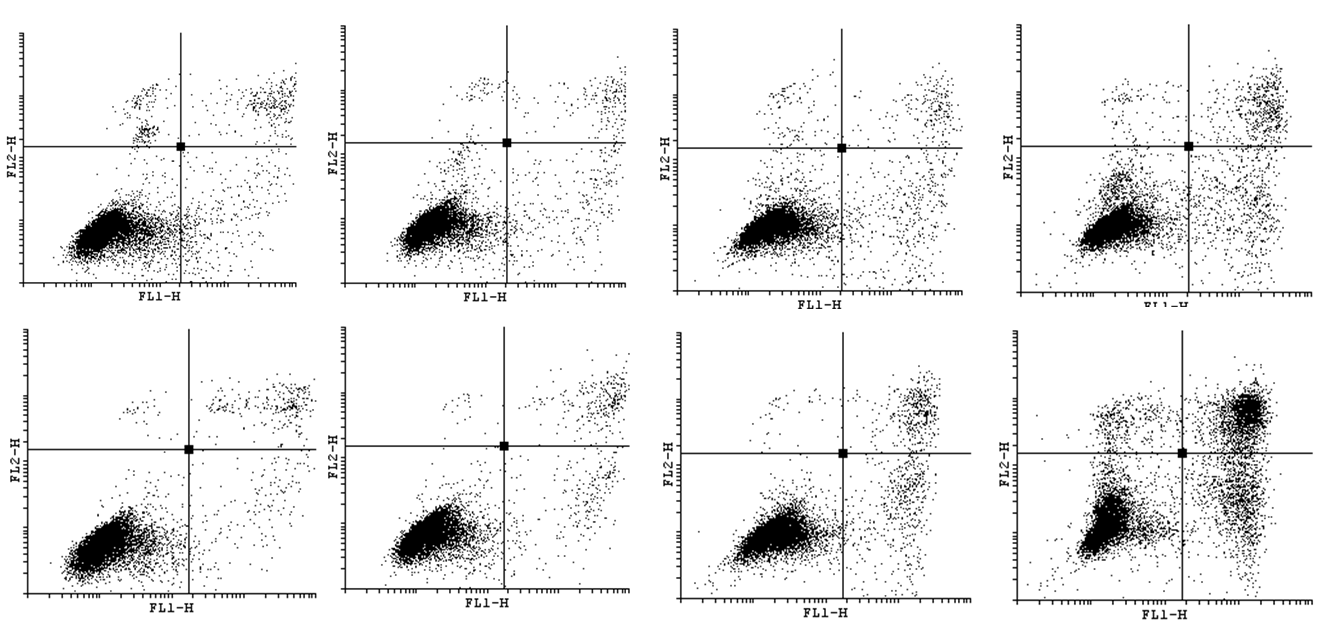
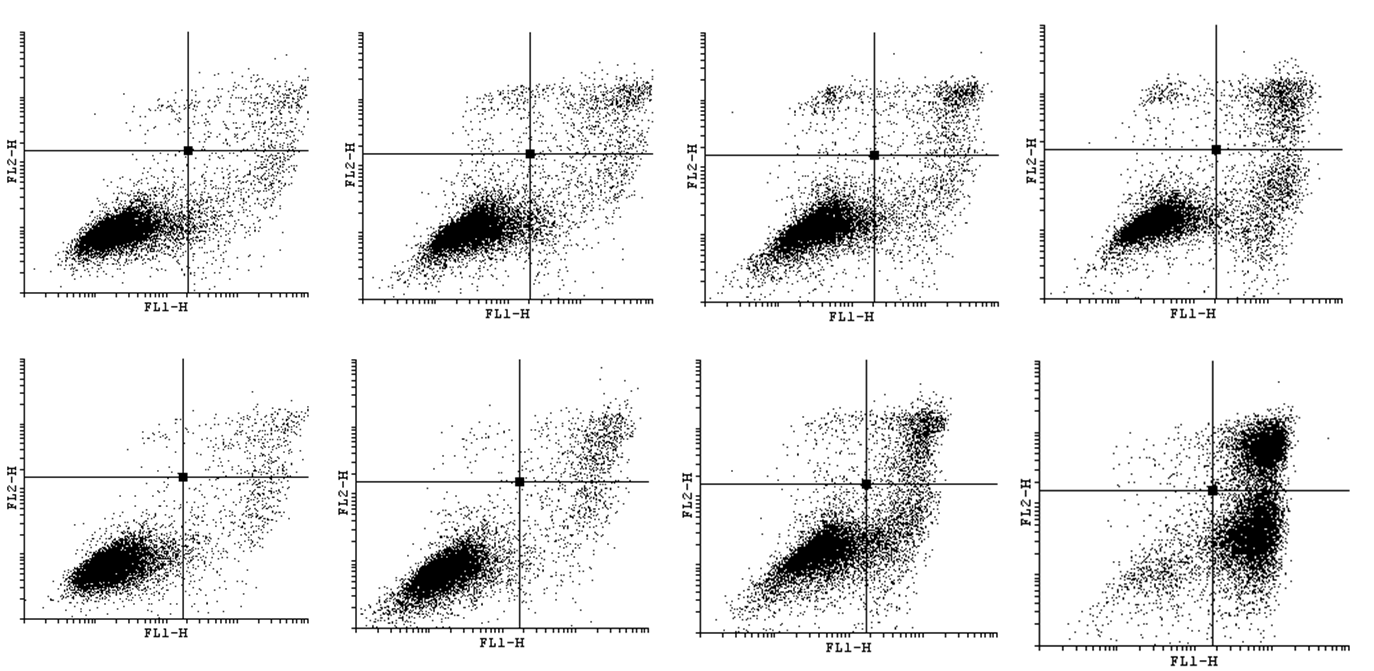
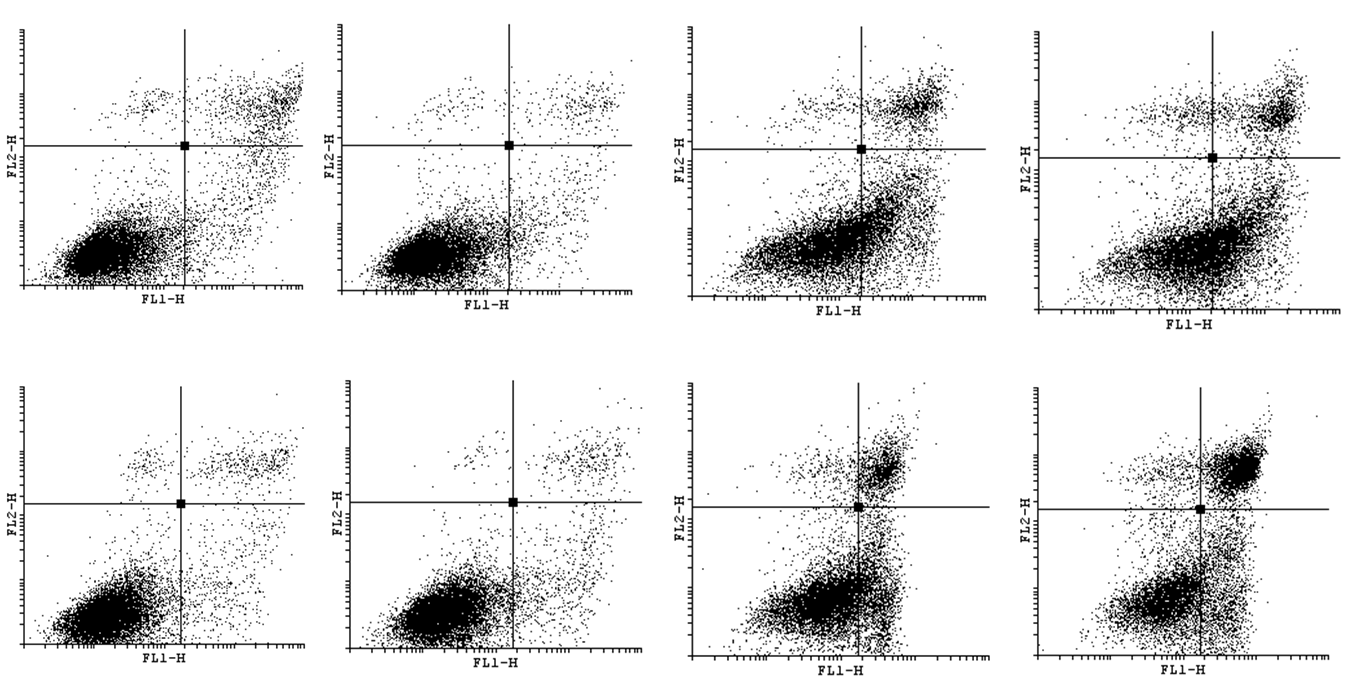
| - | In order to bring under scrutiny changes in the cell cycle we | + | In order to bring under scrutiny changes in the cell cycle we have carried out Cell Cycle assay. We observed that there occur disturbances in the cell cycle depending on concentration of acrylamide and incubation time. |
<gallery widths=800px heights=400px perrow=1 caption="Histograms for Cell Cycle assay"> | <gallery widths=800px heights=400px perrow=1 caption="Histograms for Cell Cycle assay"> | ||
| Line 76: | Line 73: | ||
<html> | <html> | ||
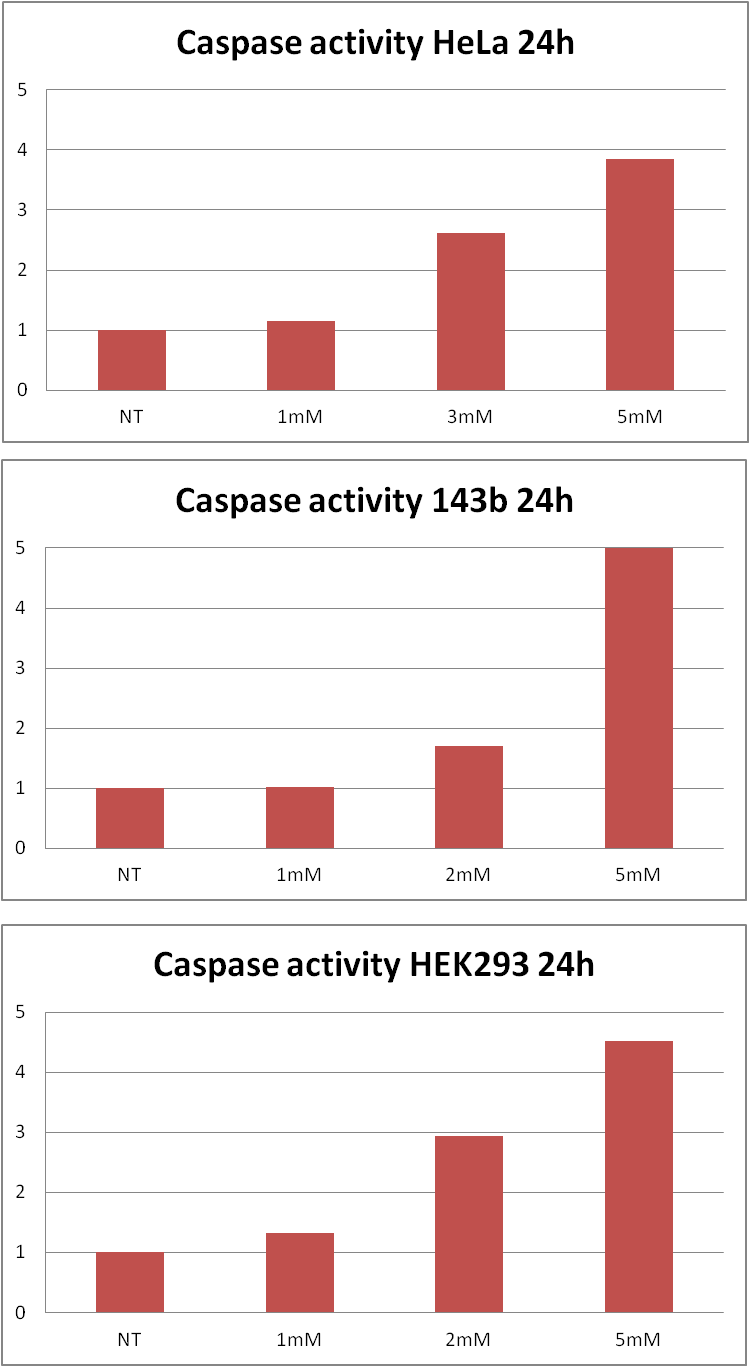
| - | These results made us to consider further investigation. As we wanted to state whether acrylamide induces apoptotic cell death we decided to measure caspase activity and examine presence of the cells that would bind | + | These results made us to consider further investigation. As we wanted to state whether acrylamide induces apoptotic cell death we decided to measure caspase activity and examine presence of the cells that would bind annexin V (which are hallmarks of apoptosis). |
| - | <a href="http://pl.promega.com/products/cell-health-and-metabolism/apoptosis-assays/luminescent-caspase-assays/caspase_glo-3_7-assay-systems/?origUrl=http%3a%2f%2fwww.promega.com%2fproducts%2fcell-health-and-metabolism%2fapoptosis-assays%2fluminescent-caspase-assays%2fcaspase_glo-3_7-assay-systems%2f" target="_blank">Caspase-Glo 3/7 Assay</a> revealed that there is an increase in caspase activity caused by acrylamide | + | <a href="http://pl.promega.com/products/cell-health-and-metabolism/apoptosis-assays/luminescent-caspase-assays/caspase_glo-3_7-assay-systems/?origUrl=http%3a%2f%2fwww.promega.com%2fproducts%2fcell-health-and-metabolism%2fapoptosis-assays%2fluminescent-caspase-assays%2fcaspase_glo-3_7-assay-systems%2f" target="_blank">Caspase-Glo 3/7 Assay</a> revealed that there is an increase in caspase activity caused by acrylamide treatment. |
| - | The Flow Cytometry analysis showed that there is a growth in | + | The Flow Cytometry analysis showed that there is a growth in annexin V positive cells number due to addition of acrylamide. |
</html> | </html> | ||
| - | [[File:caspase1.png|frameless|border|caption|450px]] | + | [[File:caspase1.png|center|frameless|border|caption|450px]] |
| Line 105: | Line 102: | ||
Despite the fact that it is impossible to relate directly the results obtained for the cell lines to human organism, it may give a basis for further examination and extended research. In our experiments '''we showed the existence of cytotoxic and cytostatic effect of acrylamide on HeLa, 143B and 293 cell lines. We also found a correlation between time of exposure to acrylamide and the decrease in cell viability and proliferation.''' | Despite the fact that it is impossible to relate directly the results obtained for the cell lines to human organism, it may give a basis for further examination and extended research. In our experiments '''we showed the existence of cytotoxic and cytostatic effect of acrylamide on HeLa, 143B and 293 cell lines. We also found a correlation between time of exposure to acrylamide and the decrease in cell viability and proliferation.''' | ||
| - | By carrying out our cytotoxicity study '''we | + | By carrying out our cytotoxicity study '''we would like to emphasize the importance of synthetic biology research'''. After showing the possible harmful effects of acrylamide we aimed to reveal how products of synthetic biology may influence and facilitate our everyday life. We also tried to raise the awareness of the threats connected with the consumption of tertiary processed foods. |
Finally, let us make you imagine that you are able to check the amount of acrylamide before eating your chips. With our FluoSafe sensor it will be soon possible. | Finally, let us make you imagine that you are able to check the amount of acrylamide before eating your chips. With our FluoSafe sensor it will be soon possible. | ||
| + | |||
| + | |||
| + | '''Bibliography:''' | ||
| + | # Stott-Miller, M., Neuhouser, ML. & Stanford, JL. Consumption of deep-fried foods and risk of prostate cancer. Epub (2013). | ||
| + | # Ehlers, A., Lenze, D., Broll, H., Zagon, J., Hummel, M. & Lampen, A. Dose dependent molecular effects of acrylamide and glycidamide in human cancer cell lines and human primary hepatocytes. Epub (2012). | ||
| + | # Besaratinia, A. & Pfeifer, GP. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28, 519–528 (2007). | ||
| + | # O'Brien, J., Wilson, I., Orton, T. & Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 28. 5421-6 (2000). | ||
| + | # Koyamaa, N., Sakamoto, H., Sakuraba, M., Koizumi, T., Takashima, Y., Hayashi, M., Matsufuji, H., Yamagata, K., Masudac, S., Kinae, S. & Honmaa M. Genotoxicity of acrylamide and glycidamide in human lymphoblastoid TK6 cells. Mutation Research 603, 151–158 (2006). | ||
{{:Team:Warsaw/Templates/StandardPageEnd}} | {{:Team:Warsaw/Templates/StandardPageEnd}} | ||
Latest revision as of 00:11, 5 October 2013
Cytotoxicity study
Contents |
Overview
The neurotoxic effects of acrylamide have been widely known for many years. Local symptoms of acrylamide poisoning such as mucous membrane and skin irritation have been formerly reported. There also exists a systemic effect characterized by fatigue, sleepiness and memory difficulties; in extreme cases also hallucination, disorientation and confusion were observed.
While acrylamide was perceived as a severe neurotoxin, there had long not been enough studies on its possible carcinogenicity. Yet, the recent research have shown that acrylamide may be engaged in processes.
Due to the rapid development of the plastic industry, acrylamide is becoming even more widely present in our environment. But the manufacturing area is not the only way by which acrylamide appears in our proximity. While deep-frying or baking, through a chemical reaction between an amino acid and a reducing sugar (called the Maillard reaction), acrylamide is formed.
Possible ways of acrylamide absorption are as follows: inhalation, ingestion, and through the skin and mucous membranes. Basing on a report of WHO from 2002 average daily intake for the general population ranges from 0.3 to 0.8 micrograms of acrylamide per kilogram of body mass.
Increased consumption of deep-fried food and, subsequently, increased intake of acrylamide, may have serious health consequences. Recent studies have revealed an association between consumption of deep-fried foods and increased prostate cancer risk. What is also suggested, is that there is positive correlation between acrylamide intake and increased risk of endometrial and ovarian cancer.
Considering the alarming effects of acrylamide exposure, we have decided to include in our project a cytotoxicity study. Our aim is to show how acrylamide may affect various cell lines originating from different tissues. In our experiments we are working on HeLa, 293 and 143B cell lines representing cervical cancer, human embryonic kidney and bone osteosarcoma cells respectively.
In our experiments we are implementing the following methods: Flow Cytometry, AlamarBlue assay, Cell Growth Assay, Cell Cycle assay and Caspase activity assay.
To indicate cell viability, we have decided to implement the Alamar Blue assay. It bases on the ability of living cells to convert nonfluorescent resazurin to the highly fluorescent molecule - resorufin. While entering the cells, resazurin is being reduced to resorufin and the bright, red fluorescence is observed. Viable cells are continuing to convert resazurin to resorufin, so the fluorescence intensity increases proportionally, which can be use to measure viability of the cells.
Flow cytometry enables us to conduct more precise analysis of chosen aspects of cell biology. After conducting flow cytometry, we will be able to state whether treated cells are undergoing apoptosis, necrosis or if the observed metabolic effects are only cytostatic.
In order to state how acrylamide may influence the progression of cell cycle we have decided to implement Cell Cycle Assay. This method employs flow cytometry to mesure number of cells in a given phase of the cell cycle. Permeabilization of cells with cold ethanol and addition of propidium iodide (PI) prior to analysis, allows us to measure the fluorescence. PI binds to DNA quantitatively so the fluorescence intensity of the stained cells is correlated with the amount of their DNA. Thus, basing on the quantity of DNA we are enabled to assign cells to different cell cycle phases.
We expect to observe the cytotoxic effects in higher acrylamide concentrations i.e. 3-5 mM. In lower concentrations <1 mM may occur the elimination of the toxicant, whose effects are to be examined.
Results
After conducting our experiments we have obtained following results:
- loss of metabolic activity (results obtained from AlamarBlue assay)
- induction of apoptotic cell death (stated by the presence of annexinV positive cells and the observation of caspase activity)
- suppression of cell growth and proliferation (stated on the basis of Cell Growth assay and AlamarBlue assay)
- disturbances in the cell cycle (based on the outcomes of Cell Cycle assay)
Getting the experiments underway
As a starting point for our experiments we have decided to implement AlamarBlue assay. It bases on the ability of living cells to convert nonfluorescent resazurin to the highly fluorescent molecule- resorufin. While entering the cells, resazurin is being reduced to resorufin and the bright, red fluorescence is observed. Viable cells are continuing to convert resazurin to resorufin, so the fluorescence intensity increases proportionally and can be use to measure viability of the cells. AlamarBlue assay provides reliable and repeatable results yet is easy to conduct thus it serves as a trustworthy basis for further research.
In order to obtain reliable results of experiments we have decided to conduct preliminary assays to determine plating density and concentration of acrylamide for each cell line: HeLa, 293 and 143B. The protocol of the assay as well as detailed conduct can be found in the section: Extras.
The outcome of AlamarBlue assay showed the existence of strong, negative correlation between acrylamide exposure and cell viability (Figure 1.). So the longer exposure time and the higher concentration of acrylamide, the weaker the cell viability.
In order to state whether the loss of cell viability, that we have observed, was caused by decrease in cell division (the cytostatic effect of acrylamide) or by cell death (the cytotoxic effect of acrylamide) we have implemented Cell growth assays. To conduct the assays we have seeded cells from different cell lines (HeLa, 293 and 143B) in 6-wells plates adding proper concentration of acrylamide to each well and counted the amount of cells in counting chamber after trypan blue staining, for 24, 48 and 72 hours incubation. This approach indicated that acrylamide treatment affects cell proliferation and induces cell death.
In order to bring under scrutiny changes in the cell cycle we have carried out Cell Cycle assay. We observed that there occur disturbances in the cell cycle depending on concentration of acrylamide and incubation time.
These results made us to consider further investigation. As we wanted to state whether acrylamide induces apoptotic cell death we decided to measure caspase activity and examine presence of the cells that would bind annexin V (which are hallmarks of apoptosis). Caspase-Glo 3/7 Assay revealed that there is an increase in caspase activity caused by acrylamide treatment. The Flow Cytometry analysis showed that there is a growth in annexin V positive cells number due to addition of acrylamide.
In our study we've included Cisplatin control, whose results are to be seen in section: Notebook
All of the results as well as graphs, figures and statistics are to be seen in section: Cellular biology lab journal
Summary
Despite the fact that it is impossible to relate directly the results obtained for the cell lines to human organism, it may give a basis for further examination and extended research. In our experiments we showed the existence of cytotoxic and cytostatic effect of acrylamide on HeLa, 143B and 293 cell lines. We also found a correlation between time of exposure to acrylamide and the decrease in cell viability and proliferation.
By carrying out our cytotoxicity study we would like to emphasize the importance of synthetic biology research. After showing the possible harmful effects of acrylamide we aimed to reveal how products of synthetic biology may influence and facilitate our everyday life. We also tried to raise the awareness of the threats connected with the consumption of tertiary processed foods.
Finally, let us make you imagine that you are able to check the amount of acrylamide before eating your chips. With our FluoSafe sensor it will be soon possible.
Bibliography:
- Stott-Miller, M., Neuhouser, ML. & Stanford, JL. Consumption of deep-fried foods and risk of prostate cancer. Epub (2013).
- Ehlers, A., Lenze, D., Broll, H., Zagon, J., Hummel, M. & Lampen, A. Dose dependent molecular effects of acrylamide and glycidamide in human cancer cell lines and human primary hepatocytes. Epub (2012).
- Besaratinia, A. & Pfeifer, GP. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28, 519–528 (2007).
- O'Brien, J., Wilson, I., Orton, T. & Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 28. 5421-6 (2000).
- Koyamaa, N., Sakamoto, H., Sakuraba, M., Koizumi, T., Takashima, Y., Hayashi, M., Matsufuji, H., Yamagata, K., Masudac, S., Kinae, S. & Honmaa M. Genotoxicity of acrylamide and glycidamide in human lymphoblastoid TK6 cells. Mutation Research 603, 151–158 (2006).
 "
"