Team:Paris Saclay/Notebook/July/4
From 2013.igem.org
(Difference between revisions)
(→Lab work) |
(→Lab work) |
||
| Line 45: | Line 45: | ||
{| align="center" | {| align="center" | ||
| - | | style="width:350px;border:1px solid black;" | | + | | style="width:350px;border:1px solid black;" | [[File:PCRPS040713.jpg|right|350px]] |
| - | | style="width:350px;border:1px solid black | + | | style="width:350px;border:1px solid black;" | |
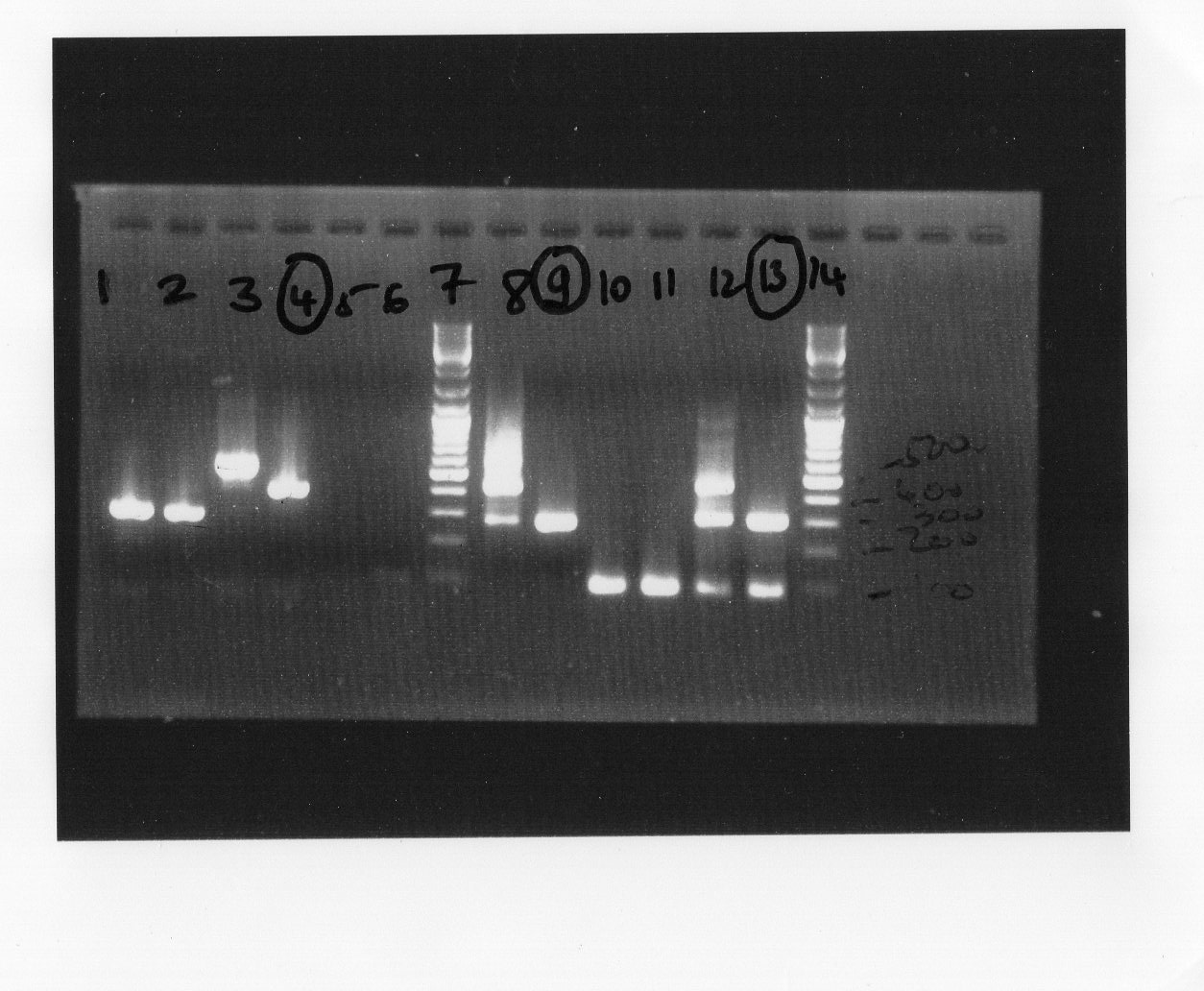
*Well 1,2,5,6,10,11 : plasmid without fnr | *Well 1,2,5,6,10,11 : plasmid without fnr | ||
*Well 3,4,8,9,12,13 : plasmid with fnr | *Well 3,4,8,9,12,13 : plasmid with fnr | ||
| Line 53: | Line 53: | ||
*Well 10 to 13 : primer fnr_up/vr | *Well 10 to 13 : primer fnr_up/vr | ||
*gel 1.5% | *gel 1.5% | ||
| - | |} | + | |}<br> |
| - | + | <p>We observed for well 10 and well 11 there were a problem which could be some fault in the manipulation. However, the well 4,9,13 conformed to our estimation.</p> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | We observed for well 10 and well 11 there were a problem which could be some fault in the manipulation. However, the well 4,9,13 conformed to our estimation. | + | |
<br> | <br> | ||
<u>Stock BBa_K1155000</u><br> | <u>Stock BBa_K1155000</u><br> | ||
| - | 1 ml of the confirmed sample mixed with 500µl glycerol. And we stocked them at -20°C. | + | <p>1 ml of the confirmed sample mixed with 500µl glycerol. And we stocked them at -20°C.</p><br> |
<u>Plasmid DNA extraction</u><br> | <u>Plasmid DNA extraction</u><br> | ||
From the cell-culture medium(2 samples plasmid with fnr), we performed some plasmid DNA extraction.<br> | From the cell-culture medium(2 samples plasmid with fnr), we performed some plasmid DNA extraction.<br> | ||
<u>Restriction digest</u><br> | <u>Restriction digest</u><br> | ||
| - | We used 3 enzymes of restriction, they were Not I, Mlu I and Hpa I. And we prepared 2 * 3 = 6 tubes. | + | <p>We used 3 enzymes of restriction, they were Not I, Mlu I and Hpa I. And we prepared 2 * 3 = 6 tubes. |
| - | So we add into each tube: | + | So we add into each tube:</p> |
| - | Extracted DNA solution : 2µl | + | *Extracted DNA solution : 2µl |
| - | Buffer Oranger : 2µl | + | *Buffer Oranger : 2µl |
| - | Enzyme : 0.5µl | + | *Enzyme : 0.5µl |
| - | H2O : 15.5µl | + | *H2O : 15.5µl |
| - | Total : 20µl | + | *Total : 20µl |
| - | The incubation was at 37°C during 90min | + | <p>The incubation was at 37°C during 90min</p> |
Revision as of 23:15, 18 September 2013
Notebook : July 4
Summary:
For regulator system:
- estimated the size of segments for bands of electrophoresis by using Clone Manager.
- Made interpretation for electrophoresis. The picture shows that the experimental result was coherent with our estimation. We constructed our first BrioBrick, BBa_K1155000 (fnr+plasmid PSB1C3).
- stored 2 colonies who contain BioBrick BBa_K1155000
- the plasmid DNA extraction was performed for BBa_K1155000.
- The extract of plasmid DNA which contain BBa_K1155000 was digested by Not I, Mlu I, Hpa I, and were amplified by PCR.
- Seeded the 4 additional broths (2 for amilCP, 2 for FNR) for plasmid extraction(mini prep).
For PCBs sensor system:
- Received the bacterial strain: pseudomonas KE707.
Lab work
- A.aero/anaerobic regulation system
- 2.BioBrick RBS+LacZ+terminator in plasmid PSB1C3
- BioBrick RBS+amilCP+terminator in plasmid PSB1C3
Electrophoresis band size estimation
We used Clonemanager for band size estimation:
| molecule | Primer pair | Size |
| Plasmid without fnr | VF/VR | 277bp |
| Plasmid with fnr | Pfnr_up/Pfnr_down | 276bp |
| Plasmid with fnr | Pfnr_up/VR | 311bp |
PCR interpretation
|
We observed for well 10 and well 11 there were a problem which could be some fault in the manipulation. However, the well 4,9,13 conformed to our estimation.
Stock BBa_K1155000
1 ml of the confirmed sample mixed with 500µl glycerol. And we stocked them at -20°C.
Plasmid DNA extraction
From the cell-culture medium(2 samples plasmid with fnr), we performed some plasmid DNA extraction.
Restriction digest
We used 3 enzymes of restriction, they were Not I, Mlu I and Hpa I. And we prepared 2 * 3 = 6 tubes. So we add into each tube:
- Extracted DNA solution : 2µl
- Buffer Oranger : 2µl
- Enzyme : 0.5µl
- H2O : 15.5µl
- Total : 20µl
The incubation was at 37°C during 90min
| Previous day | Back to calendar | Next day |
 "
"