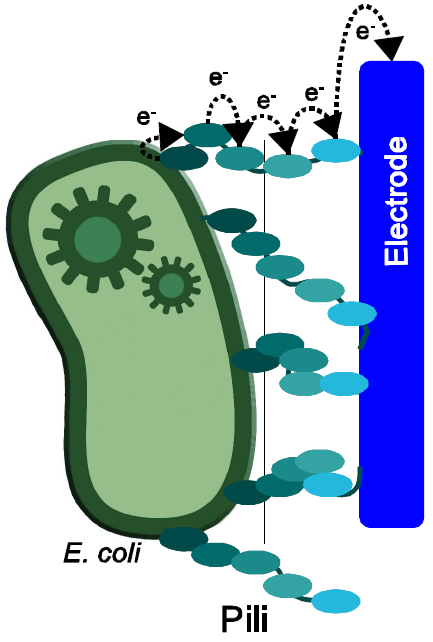
Team:Bielefeld-Germany/Biosafety/Biosafety System M
From 2013.igem.org
| Line 89: | Line 89: | ||
| - | [[File:IGEM Bielefeld 2013 biosafety Rhamnose-promoter.png]]< | + | [[File:IGEM Bielefeld 2013 biosafety Rhamnose-promoter.png|left]] |
| + | <p align="justify"> | ||
When L-rhamnose is in the milieu where E. coli is located it can be taken up by the RhaT transport system which converts it to L-rhamnulose by the isomerase RhaA. It continues by phosphorylating by the kinase RhaB in rhamnulose-1-phosphate. This is hydrolyzed by the aldolase RhaD into dihydroxyacetone phosphate and lactate aldehyde. Dihydroxyacetone is metabolized in glycolysis, and lactate aldehyde aerobe to lactate. If there are anaerobe conditions lactate aldehyde is reduced to L-1,2,-propandiol. The gene RhaBAD functions as an operon and is transcribed by RhaPBAD. Two activators, RhaR and RhaS, have to be expressed to regulate the system. This expression of these activators is in opposite direction than the expression of rhaBAD. When L-rhamnose is available RhaR binds to RhaPRS and activates the production of RhaR and RhaS. RhaS binds with L-rhamnose as an effector to RhaPBAD and RhaPT promoter and activates the transcription of the structural genes.</p> | When L-rhamnose is in the milieu where E. coli is located it can be taken up by the RhaT transport system which converts it to L-rhamnulose by the isomerase RhaA. It continues by phosphorylating by the kinase RhaB in rhamnulose-1-phosphate. This is hydrolyzed by the aldolase RhaD into dihydroxyacetone phosphate and lactate aldehyde. Dihydroxyacetone is metabolized in glycolysis, and lactate aldehyde aerobe to lactate. If there are anaerobe conditions lactate aldehyde is reduced to L-1,2,-propandiol. The gene RhaBAD functions as an operon and is transcribed by RhaPBAD. Two activators, RhaR and RhaS, have to be expressed to regulate the system. This expression of these activators is in opposite direction than the expression of rhaBAD. When L-rhamnose is available RhaR binds to RhaPRS and activates the production of RhaR and RhaS. RhaS binds with L-rhamnose as an effector to RhaPBAD and RhaPT promoter and activates the transcription of the structural genes.</p> | ||
<br> | <br> | ||
| Line 95: | Line 96: | ||
<br><br> | <br><br> | ||
| - | [[File:IGEM Bielefeld 2013 biosafety TetR.png]] | + | [[File:IGEM Bielefeld 2013 biosafety TetR.png|left]][[File:IGEM Bielefeld 2013 biosafety TetO.png|left]] |
| + | <p align="justify"> | ||
| + | The tetracyclin repressor (TetR)/ operator (TetO) originally is used by E. coli to work against the antibiotic tetracycline but in many cases it is used for regulated expression for industrial processes. When there is no tetracycline available the TetR binds with high affinity the tetracycline operator. When tetracycline is available the TetR switches his conformation and so it comes to a dissolution of the TetR and the TetO. Because of this the polymerase isn’t enhanced anymore and is able to express the genes which lies behind the TetO.</p> | ||
| + | <br> | ||
| + | [[File:IGEM Bielefeld 2013 biosafety alr.png|left]] | ||
| + | <p align="justify"> | ||
| + | The alanine-racemase alr (EC 5.1.1.1) from the gram-negative enteric bacteria ''Escherichia coli'' is a racemase, which catalyses the reversible reaction from L-alanine into the enantiomer D-alanine. For this reaction the cofactor pyridoxal-5'-phosphate (PLP) is typically needed. The constitutive alanine-racemase (''alr'') is naturally responsible for the accumulation of D-Alanin, which is an essential component of the bacterial cell wall, because it is used for the crosslinkage of the peptidoglykan. The use of D-Alanin instead of a typically L-amino acids prevents the cleavage by peptdidases, but a lack of D-Alanin leeds to a bacteristatic characteristic. So in the absence of D‑Alanine dividing cells will lyse rapidly. So if the expression of the Alanin-Racemase is repressed and there is no D-Alanine-Supplementation in the media, the cells would not increase. </p> | ||
| + | <br> | ||
| + | [[File:IGEM Bielefeld 2013 biosafety Terminator.png]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 07:57, 1 October 2013
Biosafety System TetOR alive
Overview
The tetracyclin repressor (TetR)/ operator (TetO) originally is used by E. coli to work against the antibiotic tetracycline but in many cases it is used for regulated expression for industrial processes. When there is no tetracycline available the TetR binds with high affinity the tetracycline operator. When tetracycline is available the TetR switches his conformation and so it comes to a dissolution of the TetR and the TetO. Because of this the polymerase isn’t enhanced anymore and is able to express the genes which lies behind the TetO. In our system the TetR is under the control of a rhamnose promotor (rha-promotor) which only works in the presence of rhamnose. When the bacteria would break out of the MFC there wouldn’t be enough rhamnose in the environment to activate the promotor in a way that enough TetR would be produced to block the polymerase by binding at the TetO. Therefore the polymerase binds to the promotor of TetO and it comes to the expression of RNase Ba and the degradation of the DNA.
Genetic Approach

When L-rhamnose is in the milieu where E. coli is located it can be taken up by the RhaT transport system which converts it to L-rhamnulose by the isomerase RhaA. It continues by phosphorylating by the kinase RhaB in rhamnulose-1-phosphate. This is hydrolyzed by the aldolase RhaD into dihydroxyacetone phosphate and lactate aldehyde. Dihydroxyacetone is metabolized in glycolysis, and lactate aldehyde aerobe to lactate. If there are anaerobe conditions lactate aldehyde is reduced to L-1,2,-propandiol. The gene RhaBAD functions as an operon and is transcribed by RhaPBAD. Two activators, RhaR and RhaS, have to be expressed to regulate the system. This expression of these activators is in opposite direction than the expression of rhaBAD. When L-rhamnose is available RhaR binds to RhaPRS and activates the production of RhaR and RhaS. RhaS binds with L-rhamnose as an effector to RhaPBAD and RhaPT promoter and activates the transcription of the structural genes.
The tetracyclin repressor (TetR)/ operator (TetO) originally is used by E. coli to work against the antibiotic tetracycline but in many cases it is used for regulated expression for industrial processes. When there is no tetracycline available the TetR binds with high affinity the tetracycline operator. When tetracycline is available the TetR switches his conformation and so it comes to a dissolution of the TetR and the TetO. Because of this the polymerase isn’t enhanced anymore and is able to express the genes which lies behind the TetO.
The alanine-racemase alr (EC 5.1.1.1) from the gram-negative enteric bacteria Escherichia coli is a racemase, which catalyses the reversible reaction from L-alanine into the enantiomer D-alanine. For this reaction the cofactor pyridoxal-5'-phosphate (PLP) is typically needed. The constitutive alanine-racemase (alr) is naturally responsible for the accumulation of D-Alanin, which is an essential component of the bacterial cell wall, because it is used for the crosslinkage of the peptidoglykan. The use of D-Alanin instead of a typically L-amino acids prevents the cleavage by peptdidases, but a lack of D-Alanin leeds to a bacteristatic characteristic. So in the absence of D‑Alanine dividing cells will lyse rapidly. So if the expression of the Alanin-Racemase is repressed and there is no D-Alanine-Supplementation in the media, the cells would not increase.
Results
References
- Autoren (Jahr) Titel [Link|Paper Ausgabe: Seiten].
 "
"