Team:Uppsala/results
From 2013.igem.org
| Line 282: | Line 282: | ||
<p>Although we managed to clone out and sequence verify the genes for resveratrol production, we have had some problems in the characterization. The results are unclear, and we did not have time for further investigations.For detailed information about the characterisation methods, see the protocol section </p> | <p>Although we managed to clone out and sequence verify the genes for resveratrol production, we have had some problems in the characterization. The results are unclear, and we did not have time for further investigations.For detailed information about the characterisation methods, see the protocol section </p> | ||
| - | <img id="sts_westp_uppsala" src="https://static.igem.org/mediawiki/2013/3/39/Resv_westblot_uppsala.png" | + | <div> |
| - | <i><b>Figure 1:</b>Number 2 shows a very weak band of our protein at around 43 kDA.<br> | + | <img id="sts_westp_uppsala" src="https://static.igem.org/mediawiki/2013/3/39/Resv_westblot_uppsala.png"> |
| + | |||
| + | <i class="results-fig-right"><b>Figure 1:</b>Number 2 shows a very weak band of our protein at around 43 kDA.<br> | ||
Positive control -> 1, Stilbene synthase -> 2</i> | Positive control -> 1, Stilbene synthase -> 2</i> | ||
| + | </div> | ||
| + | |||
| + | <div id="clear"></div> | ||
<b>4Cl-STS translational fusion expressed in e-coli</b> | <b>4Cl-STS translational fusion expressed in e-coli</b> | ||
<img class="results_pic_pc" id="Resveratrol_fig_1" src="https://static.igem.org/mediawiki/2013/d/dc/Resva_uppsala_tab1.png"><br> | <img class="results_pic_pc" id="Resveratrol_fig_1" src="https://static.igem.org/mediawiki/2013/d/dc/Resva_uppsala_tab1.png"><br> | ||
| - | <i><b>Figure 1:</b> E. coli supposed to produce resveratrol. As we can see, we got very low absorbance peaks at ~30 min, ~33 min and ~36 min. </i> | + | <i class="results-fig-right"><b>Figure 1:</b> E. coli supposed to produce resveratrol. As we can see, we got very low absorbance peaks at ~30 min, ~33 min and ~36 min. </i> |
<b>Resveratrol standard</b> | <b>Resveratrol standard</b> | ||
Revision as of 16:14, 2 October 2013
Results and achievement"
Achievements




Results
P-coumaric acid
We managed to clone out and biobrick tyrosine ammonia lyase and verify the biobrick by sequencing. Also we did succeful characterization on this part, showing that it works as expected. We managed to express our enzyme and detect it in a western blot, and also detect our metabolite in both spectrophotometry and hplc. The biobrick was characterized in e-coli d5halpha and e-coli nissle. For detailed information about the characterisation methods, see the protocol section.

2. TAL with Cp8 promotor
3. TAL with J23110 promotor
4. Negative control
Figure 1:SDS-page and western blot. Expression of Tyrosine ammonia lyase with constitutive promotors. The negative control is empty, showing that there is no natural protein in e-coli with the same attributes.
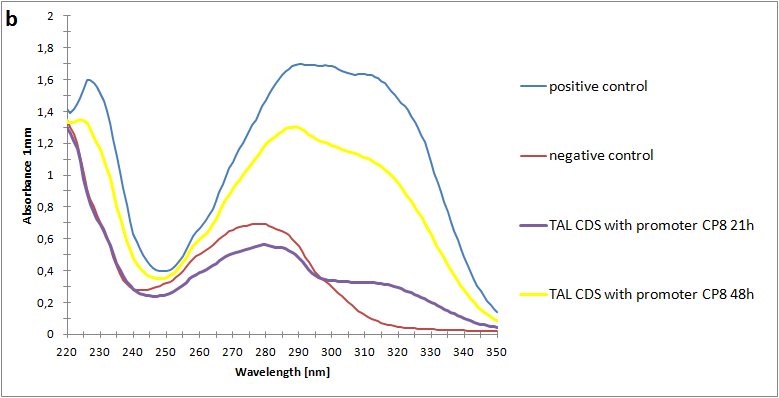
Figure 2:Absorbance spectra of extracts collected from bacterial cultures of strain pSB3K3-CP8-TAL. Samples were collected 21 h and 48 h after 30 °C incubation. Negative control is an extract from a strain with no TAL gene on the transformed plasmid. The positive control is an extract on a culture of the same strain as the negative control but with added p-coumaric acid to a concentration of 500 µM before extraction.
 | Figure 3. Graph showing the HPLC result of a sample prepared from e coli expressing tyrosine ammonia lyase. Reverse phase HPLC with a C18 matrix was used. The peak for p-coumaric acid can be seen ~9 min, as shown by the standard sample below. |
 | Figure 4. Graph showing the HPLC result of a sample standard with p-coumaric acid |
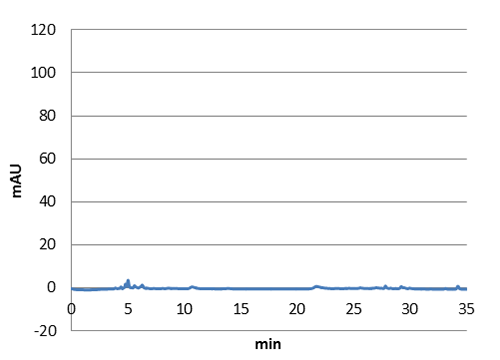 | Figure 5. E-coli culture injected to the hplc without our biobrick tyrosine ammonia lyase. Here we can see that there is originally no peak at 9 minutes. |
Resveratrol
Although we managed to clone out and sequence verify the genes for resveratrol production, we have had some problems in the characterization. The results are unclear, and we did not have time for further investigations.For detailed information about the characterisation methods, see the protocol section
 Figure 1:Number 2 shows a very weak band of our protein at around 43 kDA.
Figure 1:Number 2 shows a very weak band of our protein at around 43 kDA.Positive control -> 1, Stilbene synthase -> 2

Figure 1: E. coli supposed to produce resveratrol. As we can see, we got very low absorbance peaks at ~30 min, ~33 min and ~36 min. Resveratrol standard

Figure 2: Resveratrol standard, peaks around ~33, ~34 min. Resveratrol standard scaled
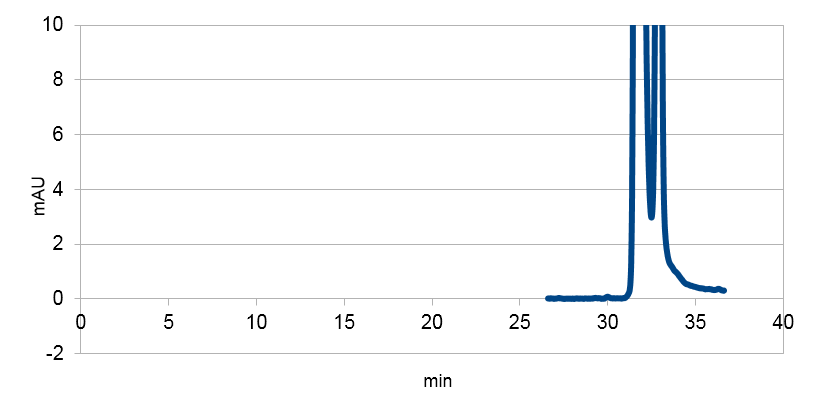
Figure 3: Resveratrol standard that is scaled down to correspond to the absorbations of our e. coli supposed to produce the corresponding metabolite. The peaks are at around ~33 and ~34. Lysed bacterial culture without plasmid of assembly

Figure 3: E. coli culture injected to the hplc without our biobrick tyrosine ammonia lyase. Here we can see that there is originally no peaks around 30-35 minutes.
Shuttle vector
Promoters
ANDERS LÄGG DIN TEXT HÄR!!
 The CP promoters strength relative to J23101 in E. coli.
The CP promoters strength relative to J23101 in E. coli.
Competition test
We grew our nissle containing the TAL biobrick together with wild type nissle (mixed 1:1) in antiobiotic free LB medium. The culture was maintaned by a thousandfold dilution in new medium every day, and letting it grow again overnight. To be able to track the fraction of each population over time, a dilution of the culture was plated on both selective, and non selective plates. The amount of colonies on the non selective plates gives us the total amount of cells, while the amount of colonies on the selective plates gives us the number of cells carrying the biobrick plasmid. The fraction of cells carrying the plasmid declined rapidly, and already after 5 days (approximately 50 generations of growth), we saw no trace of our e-coli nissle with TAL on the selective plates, while the wild type still grew on the non selective plates. This clearly shows that our genetically engineered probiotic have a hard time competing against the wild type, and if released into the environment, they would most likely be outcompeted by other bacteria within days.

 "
"









