Team:ATOMS-Turkiye/Project/Module2/Experiment
From 2013.igem.org
(→Goals:) |
(→Challenges:) |
||
| Line 4: | Line 4: | ||
| - | = | + | =Design and assembly= |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
We aim to ensure that the specifications that were drawn up are considered in the design. | We aim to ensure that the specifications that were drawn up are considered in the design. | ||
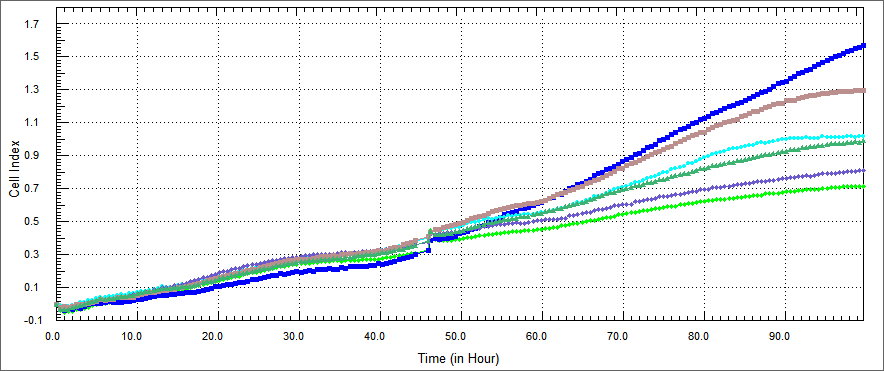
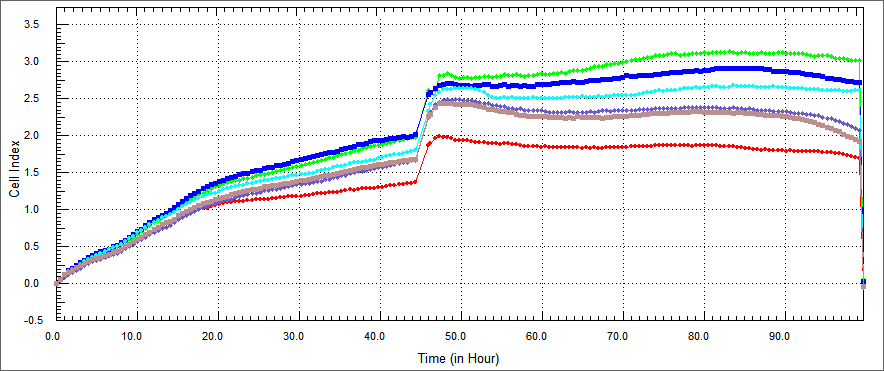
In recently studies, Apoptin has been described to induce apoptosis in various human cancer cell lines. To develop apoptin as an anti-cancer drug, efficient transduction tools have been used to deliver apoptin into tumor cells. cell-penetrating peptides, such as the trans-acting activator of transcription (TAT) protein transduction domain (PTD) and MPG peptide have therefore been used as vectors for protein delivery.So we decided apoptin fused to TAT peptide because this peptide move apoptin into cell without membrane damage.Also we fused Hemoglutinin A with TAT in order to decrease possibility of getting trapped in microcytosis.In addition to we conceived an alternative technology that offers fusing another cell penetrating peptide with apoptin, thus we constructed MPG peptide with apoptin.And we applied that idea again, on onether protein which names e4of4.E4of4 has been knew induce apoptosis as a tumor specific. We demonstrated e4of4 fused to TAT peptide as a againist tumor cells.As a result, we brought MPG-Apoptin and TAT-E4of4 to literature. | In recently studies, Apoptin has been described to induce apoptosis in various human cancer cell lines. To develop apoptin as an anti-cancer drug, efficient transduction tools have been used to deliver apoptin into tumor cells. cell-penetrating peptides, such as the trans-acting activator of transcription (TAT) protein transduction domain (PTD) and MPG peptide have therefore been used as vectors for protein delivery.So we decided apoptin fused to TAT peptide because this peptide move apoptin into cell without membrane damage.Also we fused Hemoglutinin A with TAT in order to decrease possibility of getting trapped in microcytosis.In addition to we conceived an alternative technology that offers fusing another cell penetrating peptide with apoptin, thus we constructed MPG peptide with apoptin.And we applied that idea again, on onether protein which names e4of4.E4of4 has been knew induce apoptosis as a tumor specific. We demonstrated e4of4 fused to TAT peptide as a againist tumor cells.As a result, we brought MPG-Apoptin and TAT-E4of4 to literature. | ||
| Line 66: | Line 59: | ||
Also we thougth other ways of demonstrating apoptosis, we did XTT assay. XTT assay is a colorimetric assay for the nonradioactive quantification of cellular proliferation, viability, and cytotoxicity.We measured spectrophometric analysis by ELIZA. the improved XTT method was used to study the effect of our constructs on the growth of colon, and head-neck cancer cells. The cell concentration was adjusted to 5x104 cells/mland the cells were added to a 96-well plate, at 90 µl/well. There was %10 concentrations of each constructs and each protein was set in three parallel wells with 10 µl/well. The cells were then cultured at 37˚C in a 5% (volume fraction) CO 2incubator and incubated for 48 h. XTT (5 mg/ml) was then added at 50 µl/well and the cells were cultured for 4 h. Following a period of 24, 48 or 72 h, cell viability was determined by a microplate reader at 5 nm single wavelength optical density (OD) | Also we thougth other ways of demonstrating apoptosis, we did XTT assay. XTT assay is a colorimetric assay for the nonradioactive quantification of cellular proliferation, viability, and cytotoxicity.We measured spectrophometric analysis by ELIZA. the improved XTT method was used to study the effect of our constructs on the growth of colon, and head-neck cancer cells. The cell concentration was adjusted to 5x104 cells/mland the cells were added to a 96-well plate, at 90 µl/well. There was %10 concentrations of each constructs and each protein was set in three parallel wells with 10 µl/well. The cells were then cultured at 37˚C in a 5% (volume fraction) CO 2incubator and incubated for 48 h. XTT (5 mg/ml) was then added at 50 µl/well and the cells were cultured for 4 h. Following a period of 24, 48 or 72 h, cell viability was determined by a microplate reader at 5 nm single wavelength optical density (OD) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 23:25, 16 May 2014
 "
"