Team:Bielefeld-Germany/Labjournal/September
From 2013.igem.org
| Line 163: | Line 163: | ||
**K1172001 alr (9-89-468) 163,7 ng/µL | **K1172001 alr (9-89-468) 163,7 ng/µL | ||
**K1172001 alr (9-89-468) 135,8 ng/µL | **K1172001 alr (9-89-468) 135,8 ng/µL | ||
| + | **BBa_C0040_3 250,2 ng/µL (9-108-451) | ||
| + | **BBa_C0040_4 124,5 ng/µL (9-108-452) | ||
| + | **Ptac_alr1 115,8 ng/µL (9-108-453) | ||
| + | **Ptac_alr5 68,1 ng/µL (9-108-454) | ||
| + | **Ptac_alr6 163,6 ng/µL (9-108-455) | ||
| + | **DNase Ba3 86,6 ng/µL (9-108-456) | ||
| Line 169: | Line 175: | ||
[[File:IGEM Bielefeld 2013 BIOBRICK alr.png|300px|thumb|left|In this picture is shown the 1kb DNA ladder from Thermo Scientific on the right side and the different sampes of alanine racemase and barnase. In the second pocket is the first alanine racemase biosafety biobrick. The band by 2000bp is the vector pSB1C3 and the band on 1000 bp is the lanine racemase. The pocket number 7 shows Barnase in pSB1C3. As it was cut by only one restriction enzyme it is on the higth of ca. 2350 bp. This is the second biosafety biobrick we created.]] | [[File:IGEM Bielefeld 2013 BIOBRICK alr.png|300px|thumb|left|In this picture is shown the 1kb DNA ladder from Thermo Scientific on the right side and the different sampes of alanine racemase and barnase. In the second pocket is the first alanine racemase biosafety biobrick. The band by 2000bp is the vector pSB1C3 and the band on 1000 bp is the lanine racemase. The pocket number 7 shows Barnase in pSB1C3. As it was cut by only one restriction enzyme it is on the higth of ca. 2350 bp. This is the second biosafety biobrick we created.]] | ||
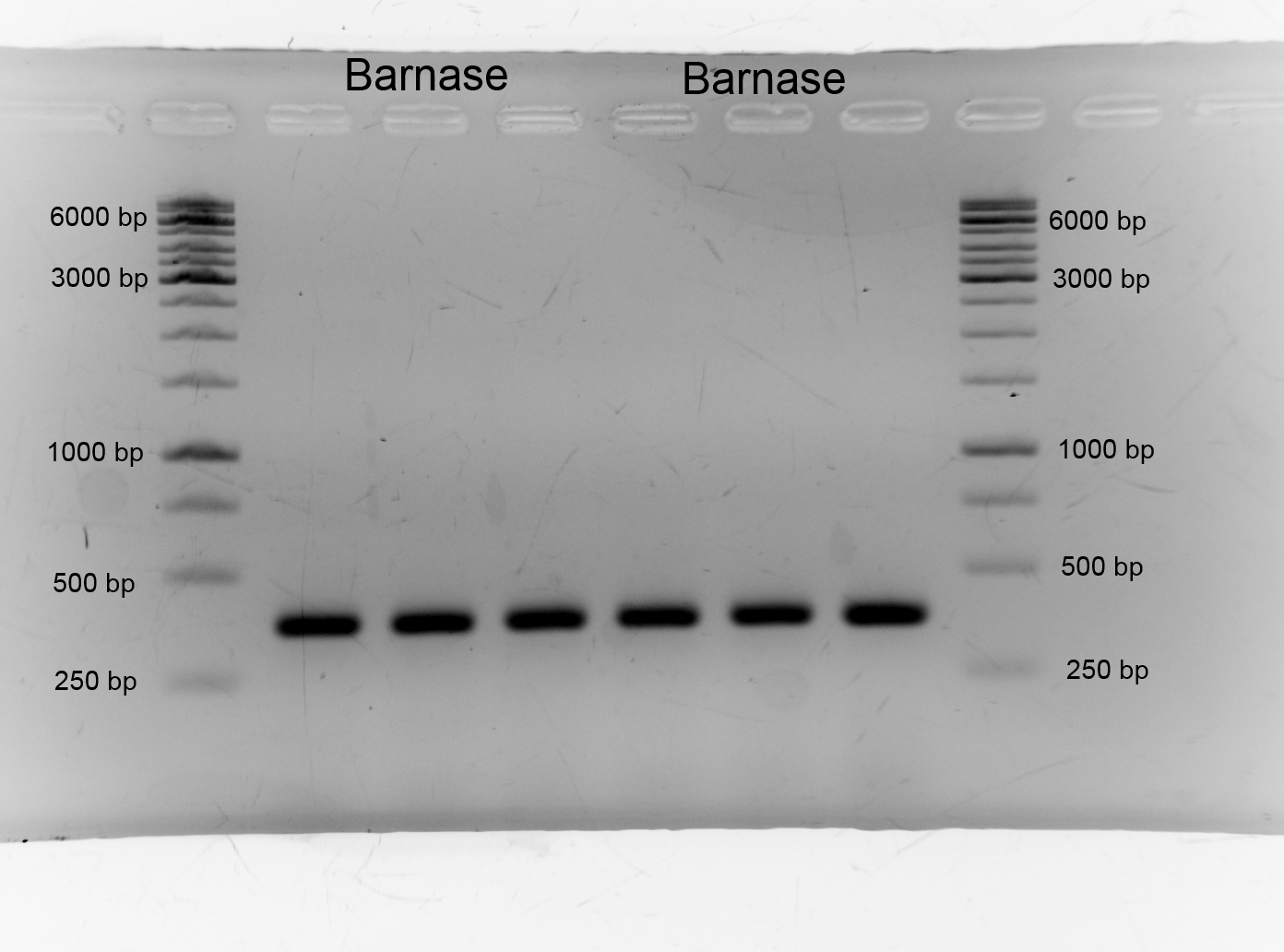
| - | [[File:IGEM Bielefeld 2013 Biosafety barnase21913.png|300px|thumb| | + | [[File:IGEM Bielefeld 2013 Biosafety barnase21913.png|300px|thumb|right|Left and right the 1kb ladder from Thermo Scientific flanks the barnase samples. The bands of the samples lie between 250bp and 500 bp as expected. The expected size of Barnase is about 330 bp. We got successfully the barnase.]] |
| - | + | ||
| + | [[File:IGEM Bielefeld 2013 Biosafety c0040ptacbarn10913.png|300px|thumb|right|Left and right the 1kb ladder from Thermo Scientific flanks the 3 different samples. In the first two pockets there is the TetR in pSB1C3 located.The expected bands by 2000bp and ca. 700 bp occured. The next three samples of ptac with alanine racemase had to be on the higth by 2000 and over 1000. This is also the fact by pocket six. The last pocket shows the Barnase in the pSB1C3 vector. Expected bands by 2000bp and ca. 350 bp are existing.]] | ||
====Porines==== | ====Porines==== | ||
Revision as of 13:54, 29 September 2013
September
Milestones
Week 18
Organization
- This week, we supported the CeBiTec pupil academy with supervising the ‘Synthetic Biology’ experiment. In addition we had a presentation about iGEM and the Bielefeld iGEM projects.
- Our team assigned the Track Food & Energy in this year, with the project title ‘Ecolectricity – currently available’. Project abstract, safety forms and team roster were added.
MFC
Mediators
- Glycerol dehydrogenase
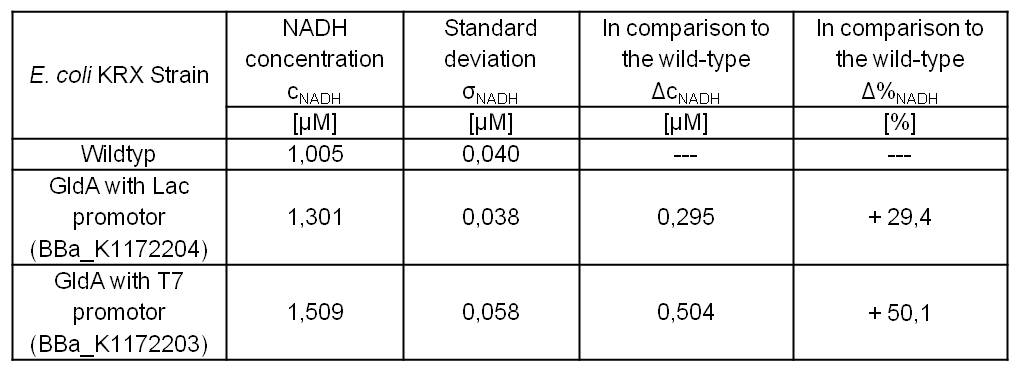
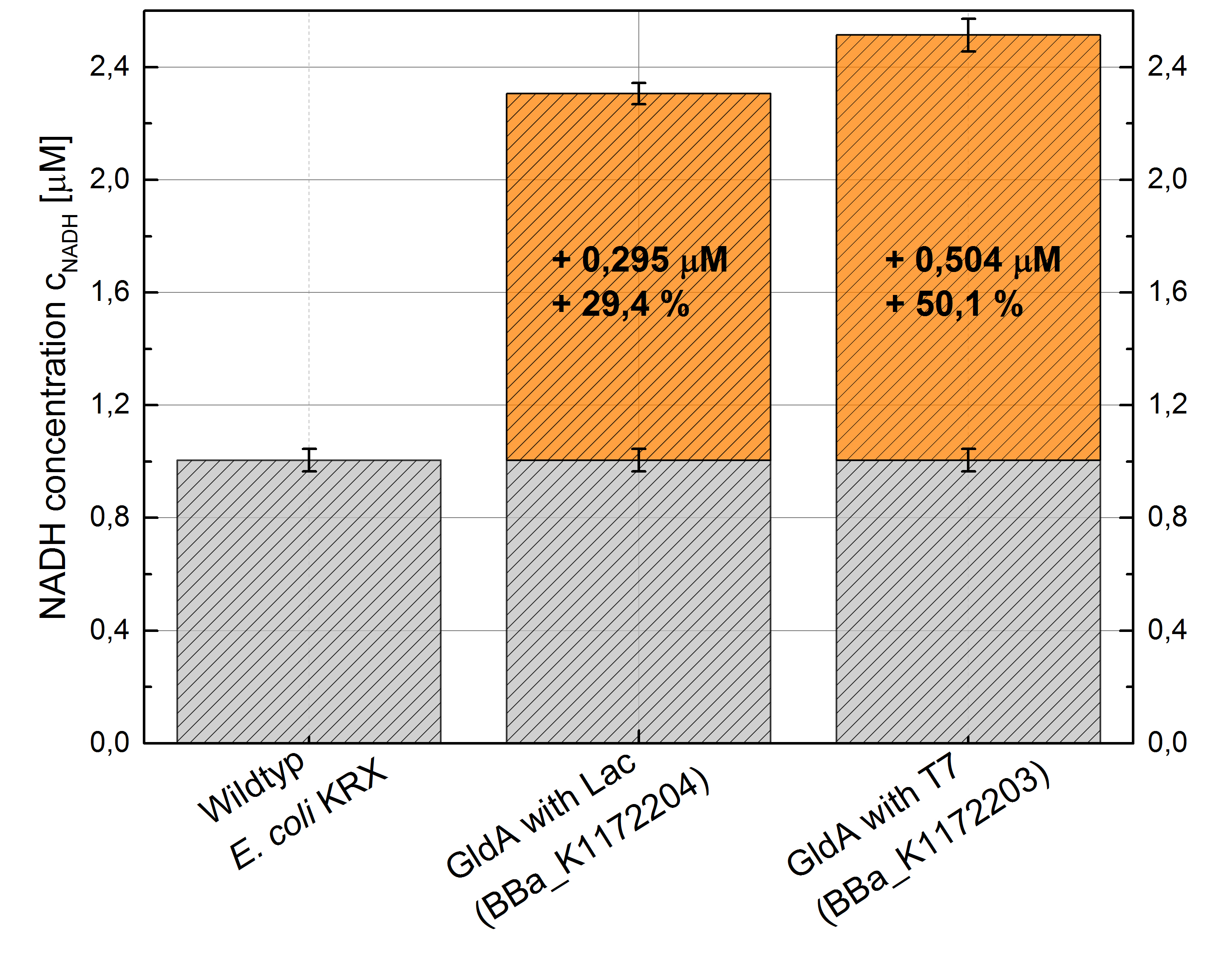
- Repeating NADH-Assay with three times PBS buffer washing step shows good results with better NADH production for E. coli KRX with pSB1C3 <bbpart>BBa_K1172203</bbpart> and <bbpart>BBa_K1172204</bbpart> compared with E. coli KRX wildtyp. GldA with T7 promotor (<bbpart>BBa_K1172203</bbpart>) shows 0,5 μM NADH overproduction and GldA with Lac promotor (<bbpart>BBa_K1172204</bbpart>) shows 0,3 μM NADH overproduction. The data refer to a culture with OD600 = 3.0.
- Second successful optimized NADH-Assay with Escherichia coli KRX Wildtyp strain and E. coli KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart>, <bbpart>BBa_K1172204</bbpart>, and <bbpart>BBa_K1172205</bbpart>.
- GldA with T7 promotor (<bbpart>BBa_K1172203</bbpart>) shows 1,7 μM NADH overproduction and GldA with Lac promotor (<bbpart>BBa_K1172204</bbpart>) shows 0,2 μM NADH overproduction. The GldA expression by the Anderson promotor (<bbpart>BBa_K1172205</bbpart>) is too weak for an efficient NADH overproduction. The data refer to a culture with OD600 = 5.0.
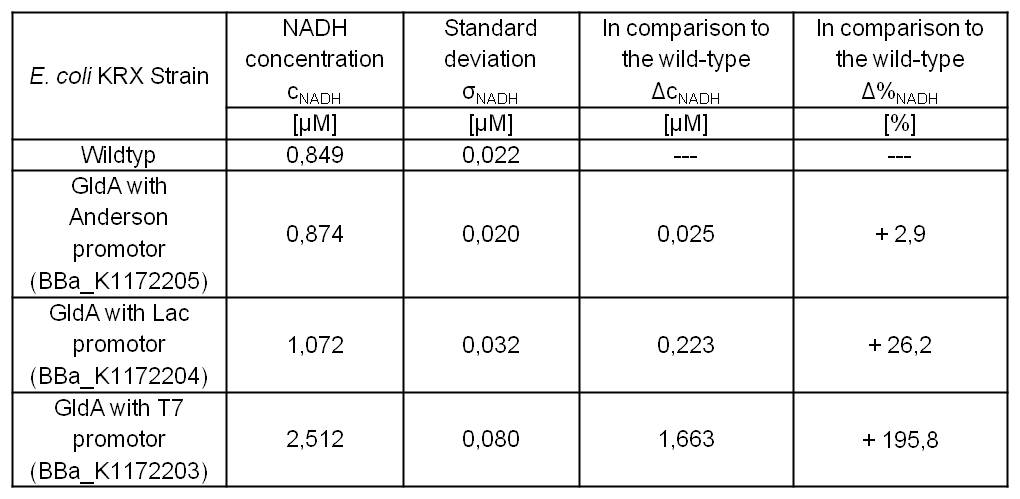
Table 2: Results of the NADH-Assay. Comparison between Escherichia coli KRX Wildtyp and Escherichia coli KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart>. NADH concentration with standard deviation and concentration in comparison to the wild-type is shown.
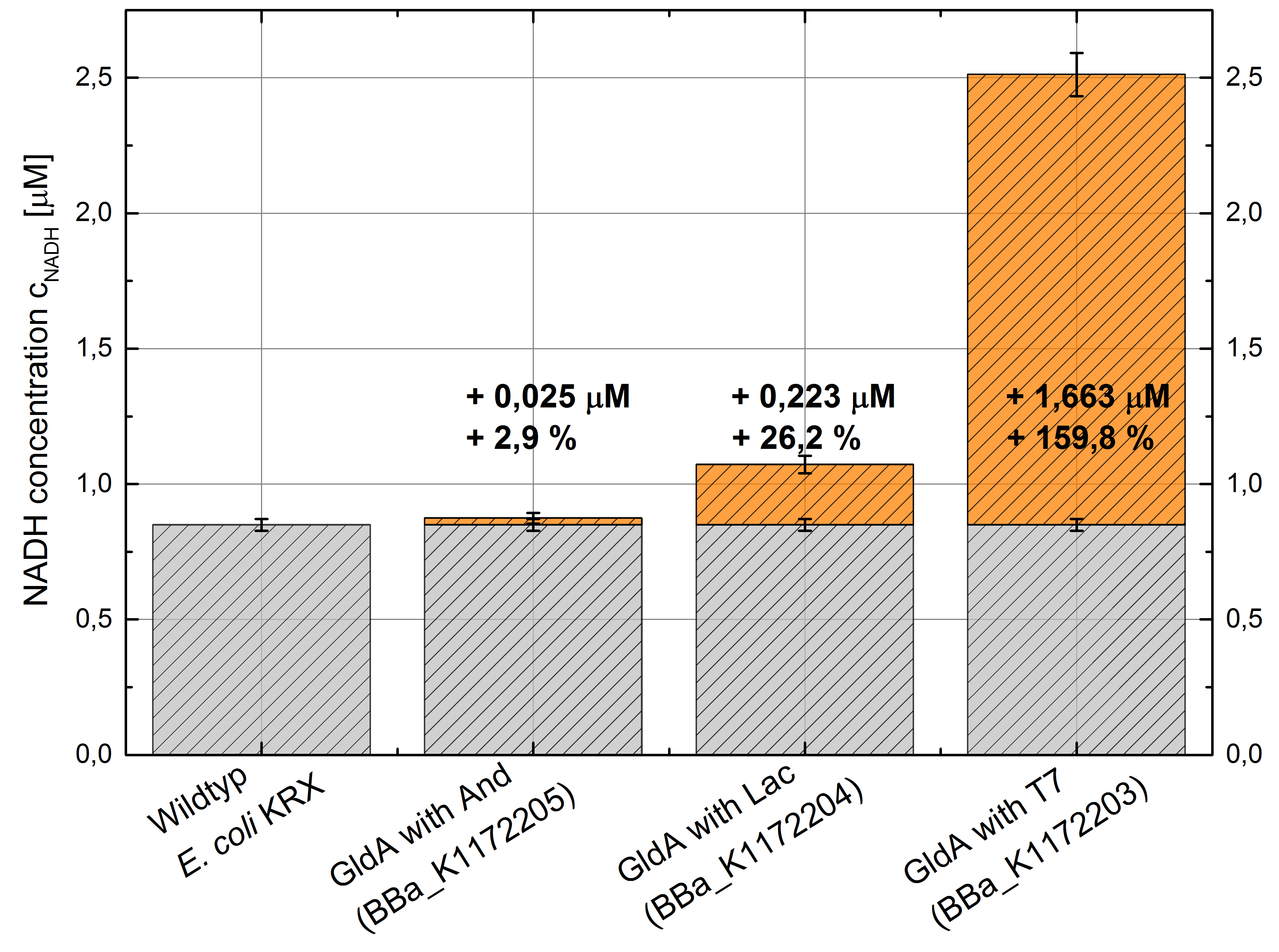
Figure 2: Column Chart with results of the NADH-Assay. Comparison between Escherichia coli KRX Wildtyp and Escherichia coli KRX with <bbpart>BBa_K1172205</bbpart>, <bbpart>BBa_K1172204</bbpart> and <bbpart>BBa_K1172203</bbpart>. NADH concentration with standard deviation and concentration in comparison to the wild-type is shown.
- Overexpressed glycerol dehydrogenase (Figure. 4 Week 17) should be examined by MALDI-TOF MS/MS.
- Tryptic digest of gel lanes for analysis with MALDI-TOF.
- Glycerol dehydrogenase was examined by MALDI-TOF MS/MS with a Mascot Score of 266 against Escherichia coli database.
- Overexpressed glycerol dehydrogenase (Figure. 4 Week 17) should be examined by MALDI-TOF MS/MS.
Cytochromes
Biosafety
- We executed the characterization of araC by cultivation (200rpm) in shaking flasks with different carbon sources. We used the M9 minimal medium with glucose/arabinose/glycin to measure the growth and the fluorescence of our biosafety system. The fluorescence is caused by the protein GFP which is a replacement for Barnase which is applied for the characterization to draw conclusions from the theoretical expression of the RNase. In continious intervals samples were taken the growth of the cells were measured by absorbance and the fluorescence by a plate reader.
Porines
- Successful Hexadecan-Assay for characterization of hydrophobicity of the outer membrane. Using Escherichia coli KRX Wildtyp and comparing it with:
- <bbpart>BBa_K1172502</bbpart>: <bbpart>BBa_K1172501</bbpart> + <bbpart>BBa_K525998</bbpart>
- <bbpart>BBa_K1172503</bbpart>: <bbpart>BBa_K1172501</bbpart> + <bbpart>BBa_J04500</bbpart>
- <bbpart>BBa_K1172504</bbpart>: <bbpart>BBa_K1172501</bbpart> + <bbpart>BBa_K608007</bbpart>
- <bbpart>BBa_K1172505</bbpart>: <bbpart>BBa_K1172501</bbpart> + <bbpart>BBa_K608006</bbpart>
- <bbpart>BBa_K1172507</bbpart>: <bbpart>BBa_K1172501</bbpart> + <bbpart>BBa_K608002</bbpart>
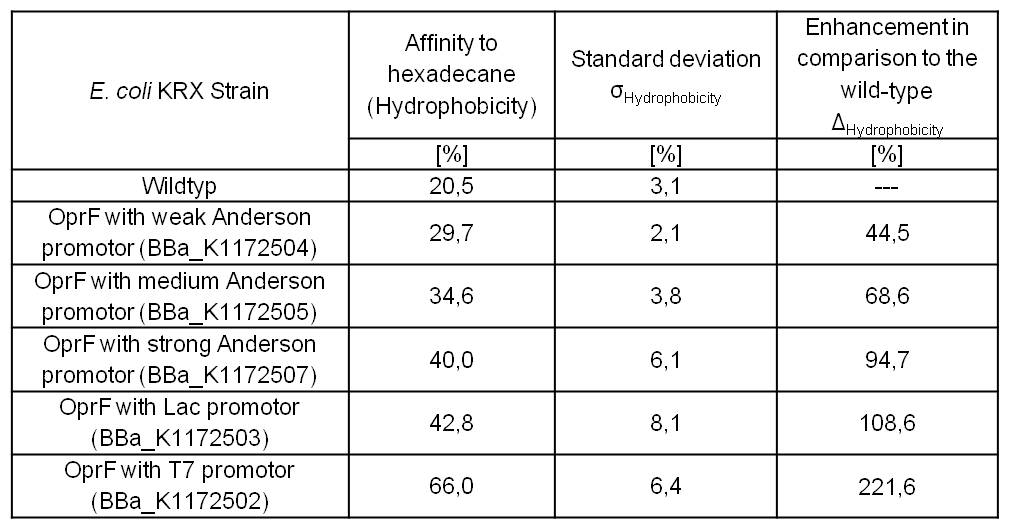
Table 3: Results of the Hexadecane-Hydrophobicity-Assay. Comparison of protein Hydrophobicity between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Affinity to hexadecane (Hydrophobicity) with standard deviation and enhancement in comparison to the wild-type is shown.
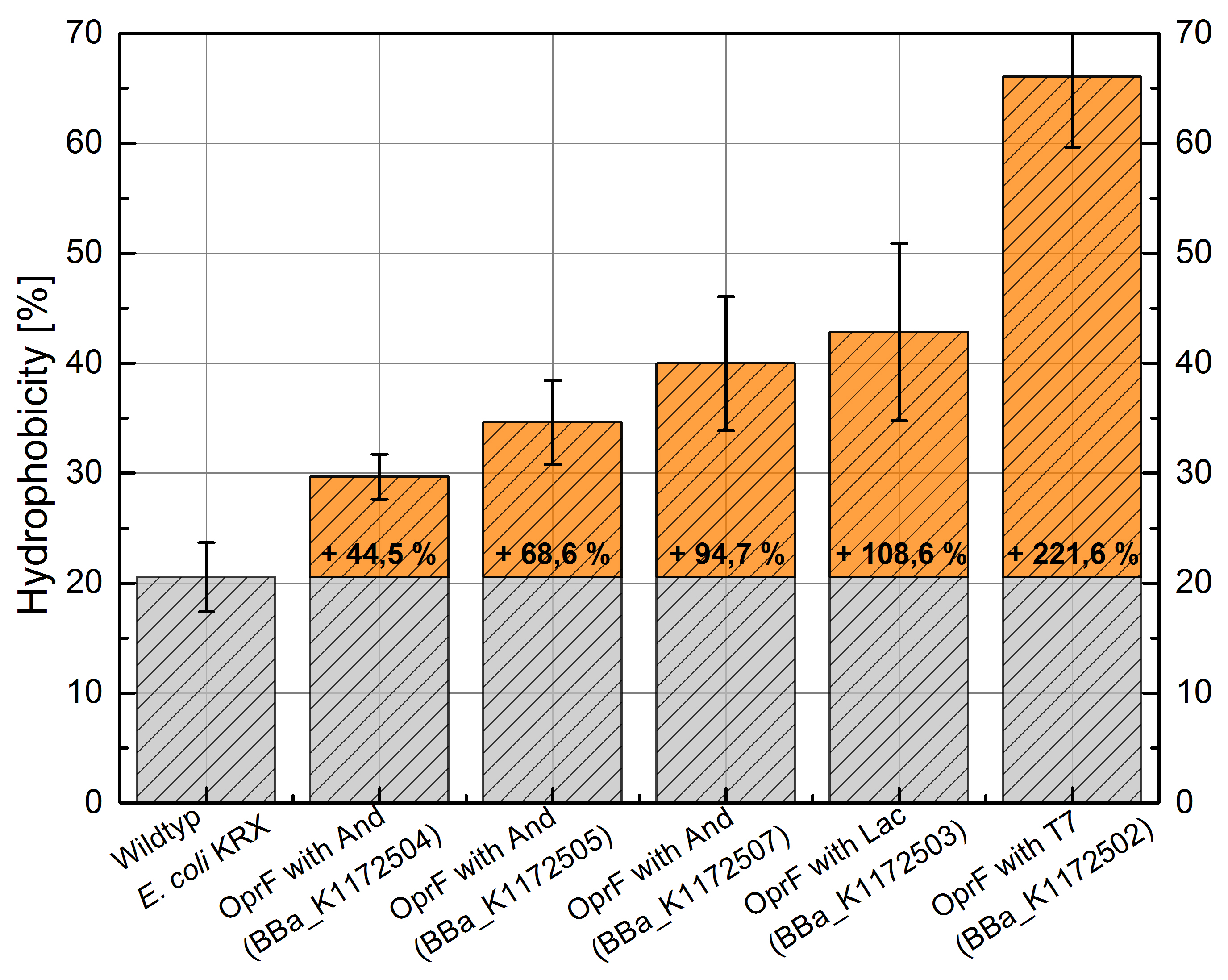
Figure 3: Results of the Hexadecane-Hydrophobicity-Assay. Comparison of protein Hydrophobicity between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Affinity to hexadecane (Hydrophobicity) with standard deviation and enhancement in comparison to the wild-type is shown.
- The hydrophobicity increases continuously with increasing promoter strength up to 221% hydrophobizity in contrast to wildtyp strain.
- Successful ONPG and NPN uptake assays were performed. Membrane permeability is continuously increasing from weak to strong promoter strength.
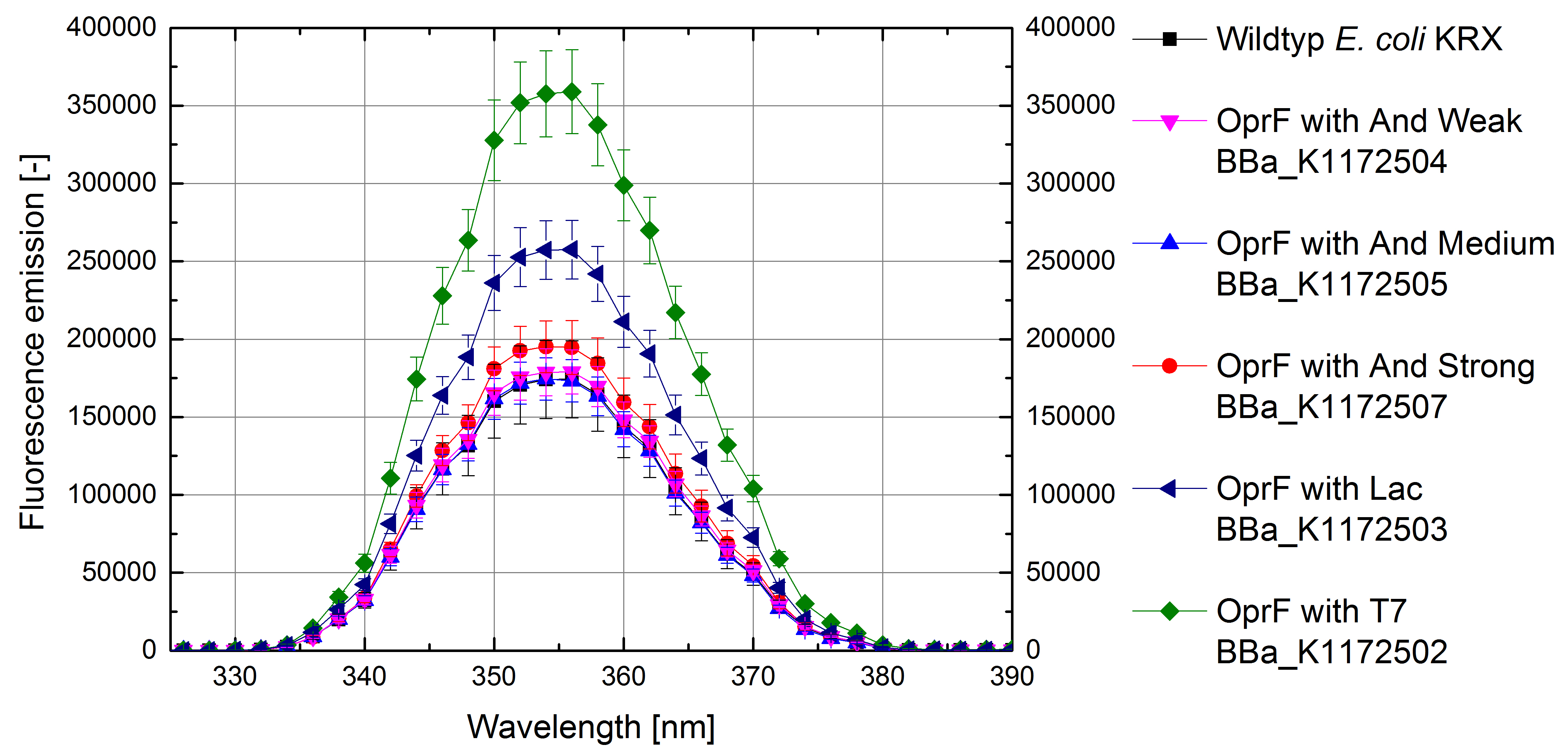
Figure 4: Results of the NPN-uptake-assay. Comparison of fluorescence emission between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Fluorescence emission scan from 320 up to 390 nm wavelength with standard deviation is shown.
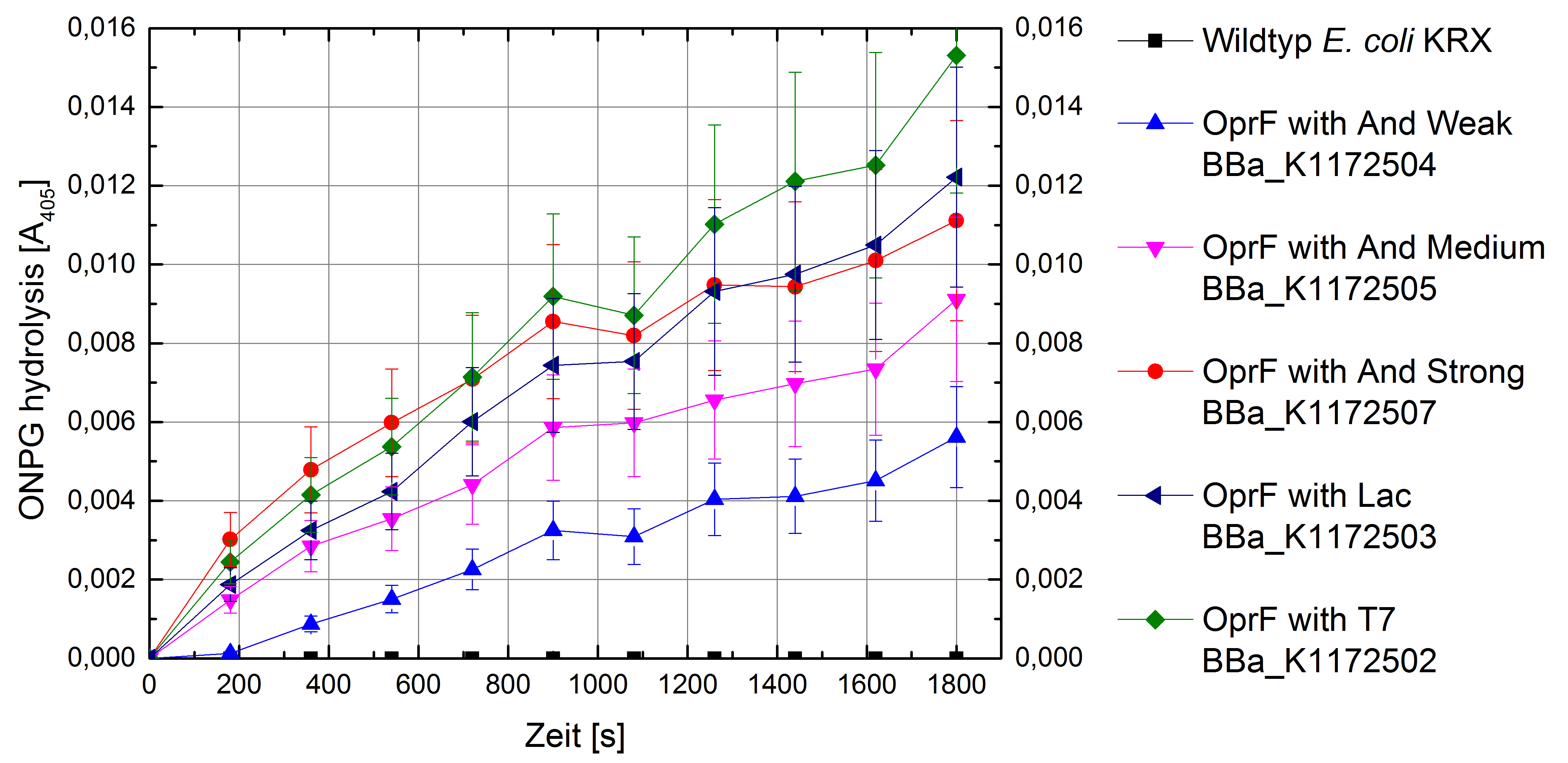
Figure 5: Results of the ONPG-uptake-assay. Comparison of ONPG hydrolysis between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart>, <bbpart>BBa_K1172504</bbpart>, <bbpart>BBa_K1172505</bbpart> and <bbpart>BBa_K1172507</bbpart>. Absorbance at 405 nm wavelength with standard deviation is shown.
- Beside testing OprF Biobrick (<bbpart>BBa_K1172501</bbpart>) with different assays and SDS-PAGE, the membrane should be visually displayed. The technique of choice is atomic force microscopy.
- The glass slides were coated with Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart> in order to compare the membrane differences. The AFM itself was performed with the help of the working group of Prof. Dr. Dario Anselmetti, with special help from Dr. Volker Walhorn.
Week 19
Organization
- Our WDR TV contribution could be seen in the ‘OWL local time’ and in the WDR library.
- We have successfully performed the day of synthetic biology in the city of Bielefeld. We were able to explain many people our project with the help of our poster and the kids had a lot of fun with performing different experiments for introducing them into scientific work. DNA isolation from fruit and vegetables, pipetting of bright colors, chromatography with markers, a potato battery and microscopy were on the program. All in all a very successful day.
MFC
Mediators
Cytochromes
Biosafety
- We isolated the plasmids which we have transformed into KRX cells days before. We also transformed the Biobricks <bbpart>BBa_R0010</bbpart> (lacI regulated promoter) and <bbpart>BBa_K741002</bbpart> (plac with RBS+GFP+terminators) and measured their DNA concentration:
- Alr_1: 214,2 ng/µL
- Alr_2: 203,6 ng/µL
- Alr_3: 301,3 ng/µL
- BBa_R0010: 121,7 ng/µL
- BBa_R0010: 136,0 ng/µL
- BBa_K741002: 145,9 ng/µL
- BBa_K741002: 175,1 ng/µL
- We deleted GFP out of the pBAD device for preparing to replace the RNase by cutting and insertion with the correlated restriction enzymes.
- PCR, purification and transformation in KRX:
- RNase Ba fwd_rev 1: 79,2 ng/µL
- RNase Ba fwd_rev 2: 85,9 ng/µL
- Ptac fwd_rev 1: 74,4 ng/µL
- Ptac fwd_rev 2: 69,8 ng/µL
- RNase_pBAD (GFP_del) 68,4 ng/µL
- Ptac_alr_pre_suf 1: 33,4 ng/µL
- Ptac_alr_pre_suf 2: 41,0 ng/µL
- We isolated more plasmids out of KRX competente cells. The new BBa were previously transformed into competent cells.
- Ptac_alr (9-89-451) 190,8 ng/µL
- Ptac_alr (9-89-452) 212,8 ng/µL
- Ptac_alr (9-89-453) 117,9 ng/µL
- Ptac_alr (9-89-454) 107,3 ng/µL
- RNase Ba (9-89-455) 101,0 ng/µL
- RNase Ba (9-89-456) 82,5 ng/µL
- pBAD_RNase Ba (9-89-4567 177,9 ng/µL
- pBAD_RNase Ba (9-89-458) 151,9 ng/µL
- pBAD_RNase Ba (9-89-459) 89,2 ng/µL
- pBAD_RNase Ba (9-89-460) 83,5 ng/µL
- BBa_I13504 (9-89-461) 152,3 ng/µL
- BBa_I13504 (9-89-462) 159,4 ng/µL
- BBa_R0040 (9-89-463) 125,7 ng/µL
- BBa_R0040 (9-89-464) 129,0 ng/µL
- BBa_R0040 (9-89-465) 105,5 ng/µL
- K1172001 alr (9-89-466) 169,8 ng/µL
- K1172001 alr (9-89-467) 156,0 ng/µL
- K1172001 alr (9-89-468) 163,7 ng/µL
- K1172001 alr (9-89-468) 135,8 ng/µL
- BBa_C0040_3 250,2 ng/µL (9-108-451)
- BBa_C0040_4 124,5 ng/µL (9-108-452)
- Ptac_alr1 115,8 ng/µL (9-108-453)
- Ptac_alr5 68,1 ng/µL (9-108-454)
- Ptac_alr6 163,6 ng/µL (9-108-455)
- DNase Ba3 86,6 ng/µL (9-108-456)
After the isolation and measuring we checked if the right insert is in the vector, so we did restriction analysis with EcoR1+ Pst1 and Barnase+pSB1C3 only with EcoR1. The results are listened below:
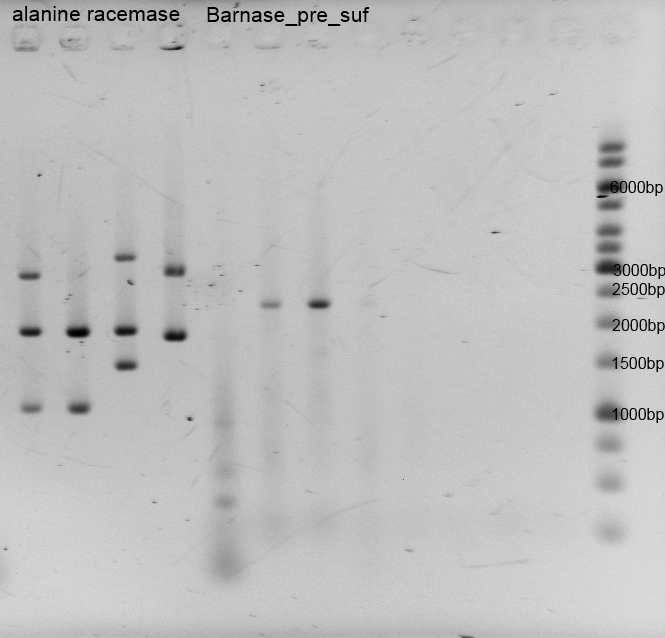
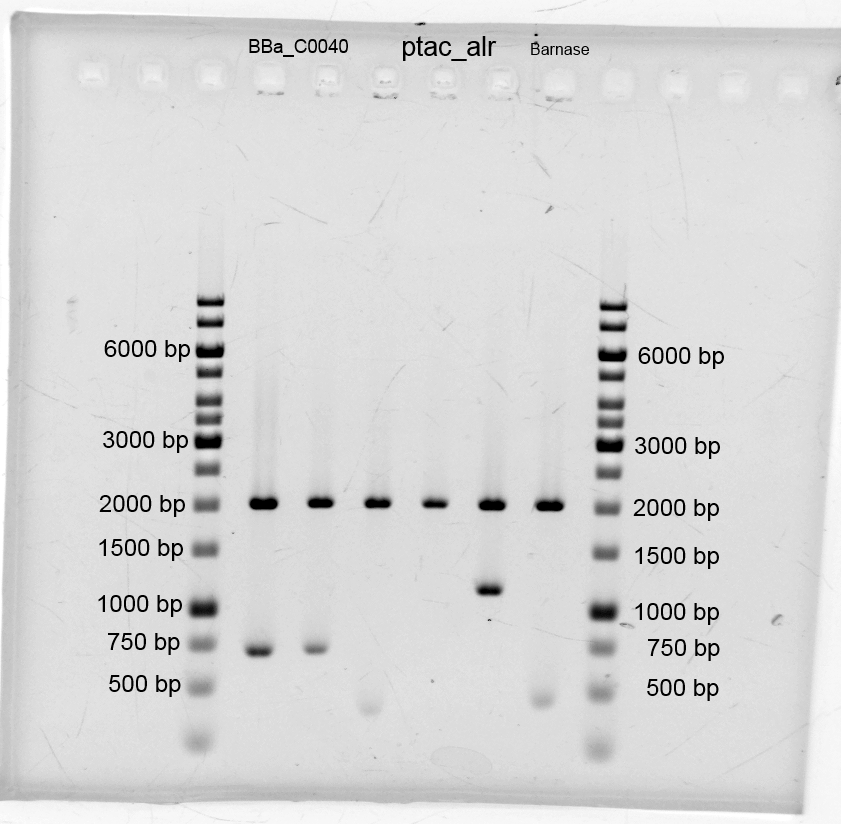
Porines
- SDS-PAGE with improved protein isolation of outer membrane porin oprF by Release of periplasmic protein fraction from E. coli by cold osmotic shock using Cell fractionating buffer 2.2. There is a slight overexpression band for E.coli with T7 promoter (<bbpart>BBa_K1172502</bbpart>) at the expected size of OprF (36 kDa). But the gel is a little bit overloaded with protein. Maybe the SDS concentration in Cell fractionating buffer 2.2 was elected with 2% too high, which has led to partial cell lysis and total protein extraction.
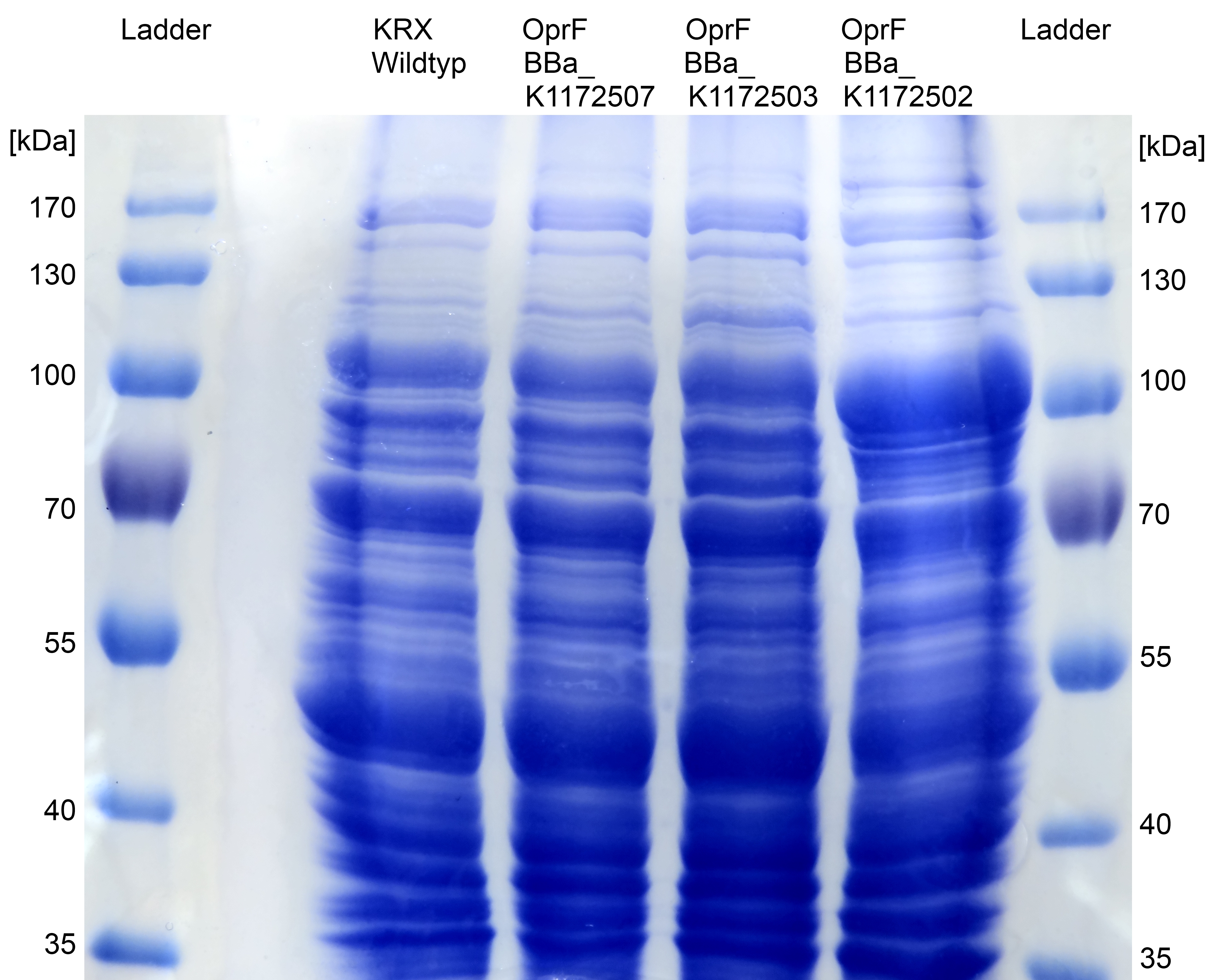
Figure 6: SDS-PAGE with Prestained Protein Ladder from Thermo Scientific as marker. Comparison of protein expression between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart> and <bbpart>BBa_K1172507</bbpart> after periplasmic protein fractioning with Cell fractioning buffer 2.2.
- Further improving of cell fractioning for protein isolation of outer membrane porin oprF by Release of periplasmic protein fraction from E. coli by cold osmotic shock using Cell fractionating buffer 2.3.
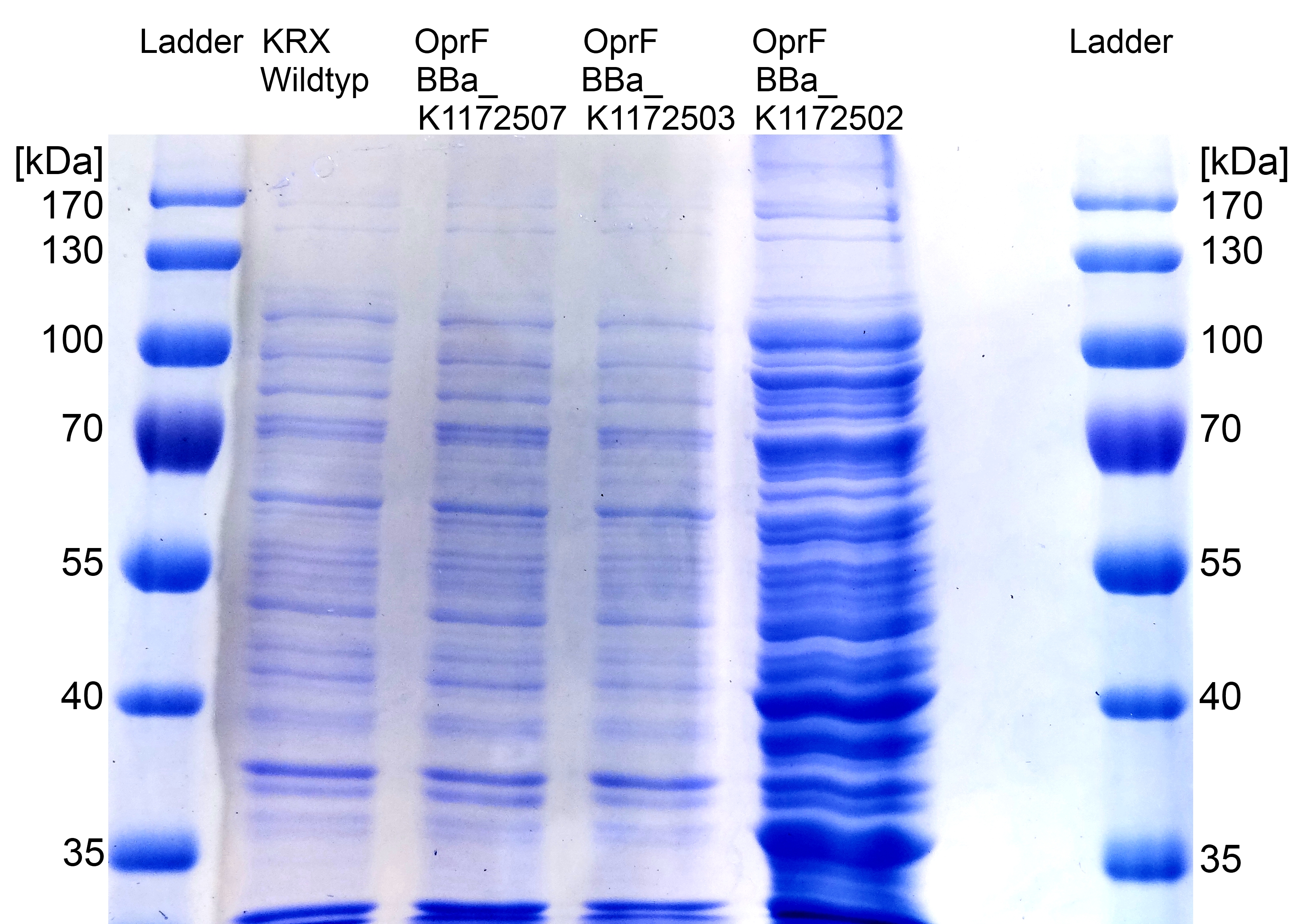
Figure 7: SDS-PAGE with Prestained Protein Ladder from Thermo Scientific as marker. Comparison of protein expression between Escherichia coli KRX wild type and Escherichia coli KRX with <bbpart>BBa_K1172502</bbpart>, <bbpart>BBa_K1172503</bbpart> and <bbpart>BBa_K1172507</bbpart> after periplasmic protein fractioning with Cell fractioning buffer 2.3.
- The SDS-PAGE shows a significantly higher protein concentration for E.coli with OprF and T7 promoter (<bbpart>BBa_K1172502</bbpart>). It seems to be that the higher membrane permeability allows a better release of membrane proteins by 0.2 % SDS. Nevertheless, we can see a strong overexpression band at the expected OprF size of about 36 kDa for <bbpart>BBa_K1172502</bbpart>, which is equated with a strong expression and overproduction of OprF.
- Overexpressed outer membrane porin (Figure. 5) should be examined by MALDI-TOF MS/MS.
- Tryptic digest of gel lanes for analysis with MALDI-TOF.
- Outer membrane porin was examined by MALDI-TOF MS/MS with a Mascot Score of 222 against bacteria database.
Week 20
Organization
- iGEM-Team Bielefeld 2013 T-shirts and hoodies were designed and are in production. Orange shirts with a great Ecolectricity logo. We are pleased to wear them in Lyon.
- As announced, we have participated at the conference '60 Years of DNA’ in Berlin at 14. September and received good reviews for our project.
MFC
Mediators
- Glycerol dehydrogenase
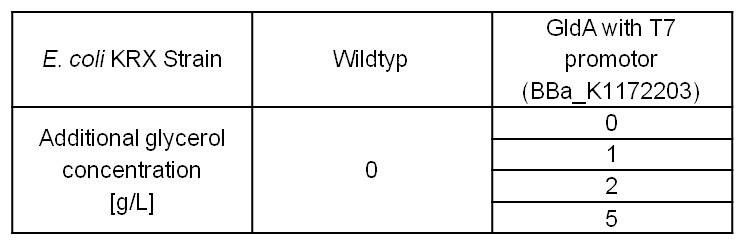
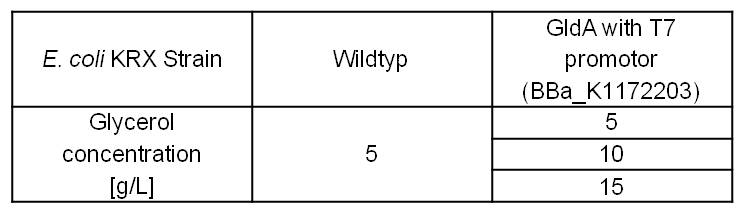
- Glycerol dependent NADH-Assay with Escherichia coli KRX Wildtyp strain and E. coli KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart> by testing supplementation of different glycerol concentrations to the LB-medium.
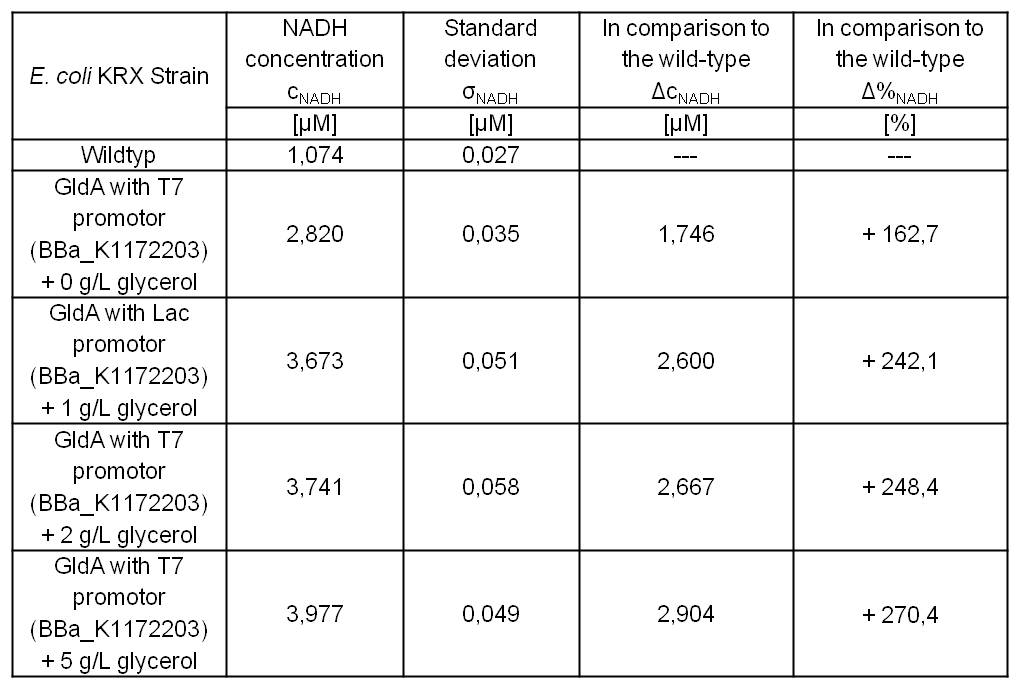
Table 5: Results of the NADH-Assay. Comparison between Escherichia coli KRX Wildtyp and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> supplemented with different amounts of glycerol to LB-medium. NADH concentration with standard deviation and concentration in comparison to the wild-type is shown.
- NADH-Assay shows a quiet good NADH overproduction for <bbpart>BBa_K1172203</bbpart> with increasing glycerol concentration.
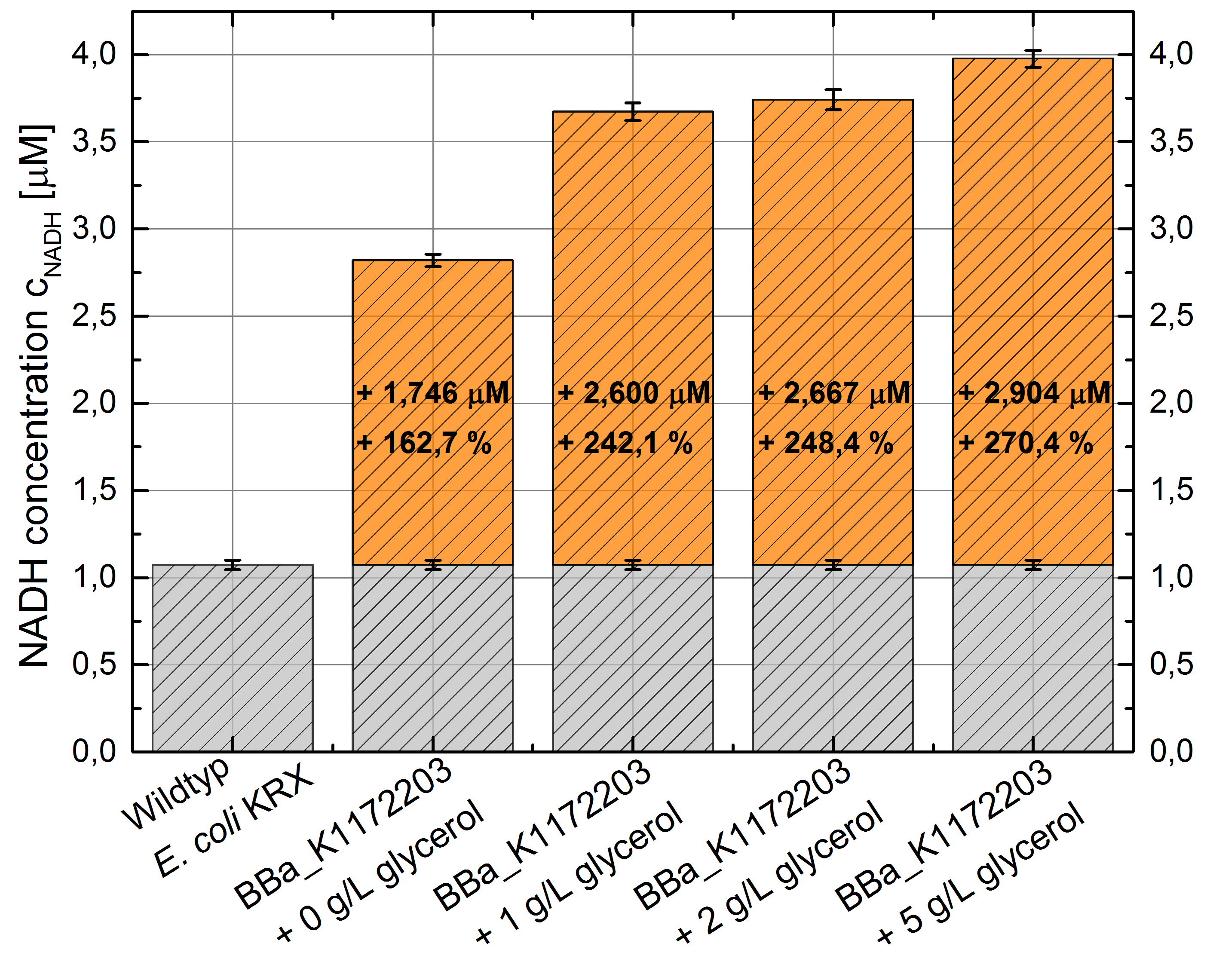
Figure 8: Column Chart with results of the NADH-Assay. Comparison between Escherichia coli KRX Wildtyp and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> supplemented with different amounts of glycerol to LB-medium. NADH concentration with standard deviation and concentration in comparison to the wild-type is shown.
Cytochromes
Biosafety
- We transformed the tetracyclin repressor into KRX via electroporation for plasmid isolation. After this we isolated the plasmids and other plasmids which are listend below.
BBa_C0040_3 250,2 ng/µL (9-108-451) BBa_C0040_4 124,5 ng/µL (9-108-452) Ptac_alr1 115,8 ng/µL (9-108-453) Ptac_alr5 68,1 ng/µL (9-108-454) Ptac_alr6 163,6 ng/µL (9-108-455) DNase Ba3 86,6 ng/µL (9-108-456)
Porines
Week 21
Organization
MFC
Mediators
- Glycerol dehydrogenase
- Glycerol dependent NADH-Assay with Escherichia coli KRX Wildtyp strain and E. coli KRX with pSB1C3 and <bbpart>BBa_K1172203</bbpart> by testing different glycerol concentrations of M9-medium.
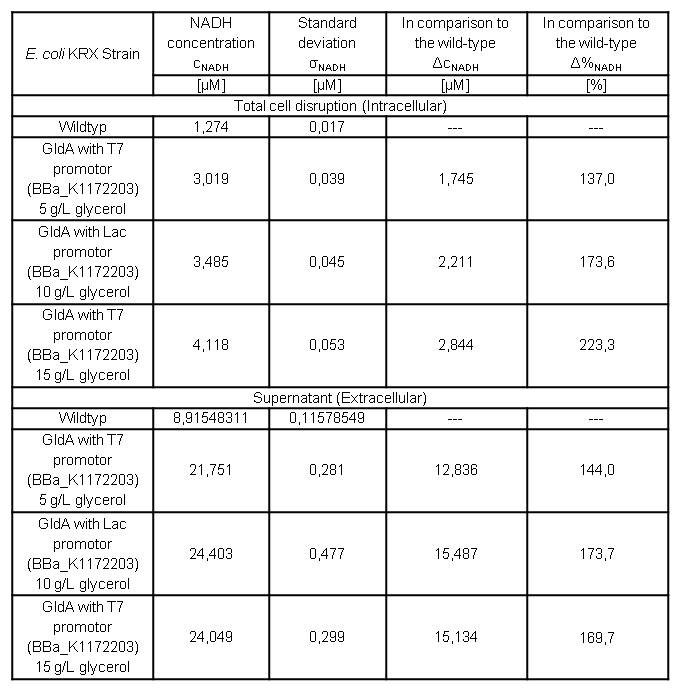
Table 7: Results of the NADH-Assay. Comparison between Escherichia coli KRX Wildtyp and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> grown in M9-medium with different concentrations of glycerol. NADH concentration with standard deviation and concentration in comparison to the wild-type is shown for cell disruption and supernatant fraction.
- NADH-Assay shows an increasing intra- and extracellular NADH concentration for <bbpart>BBa_K1172203</bbpart> with increasing glycerol concentration in M9-medium.
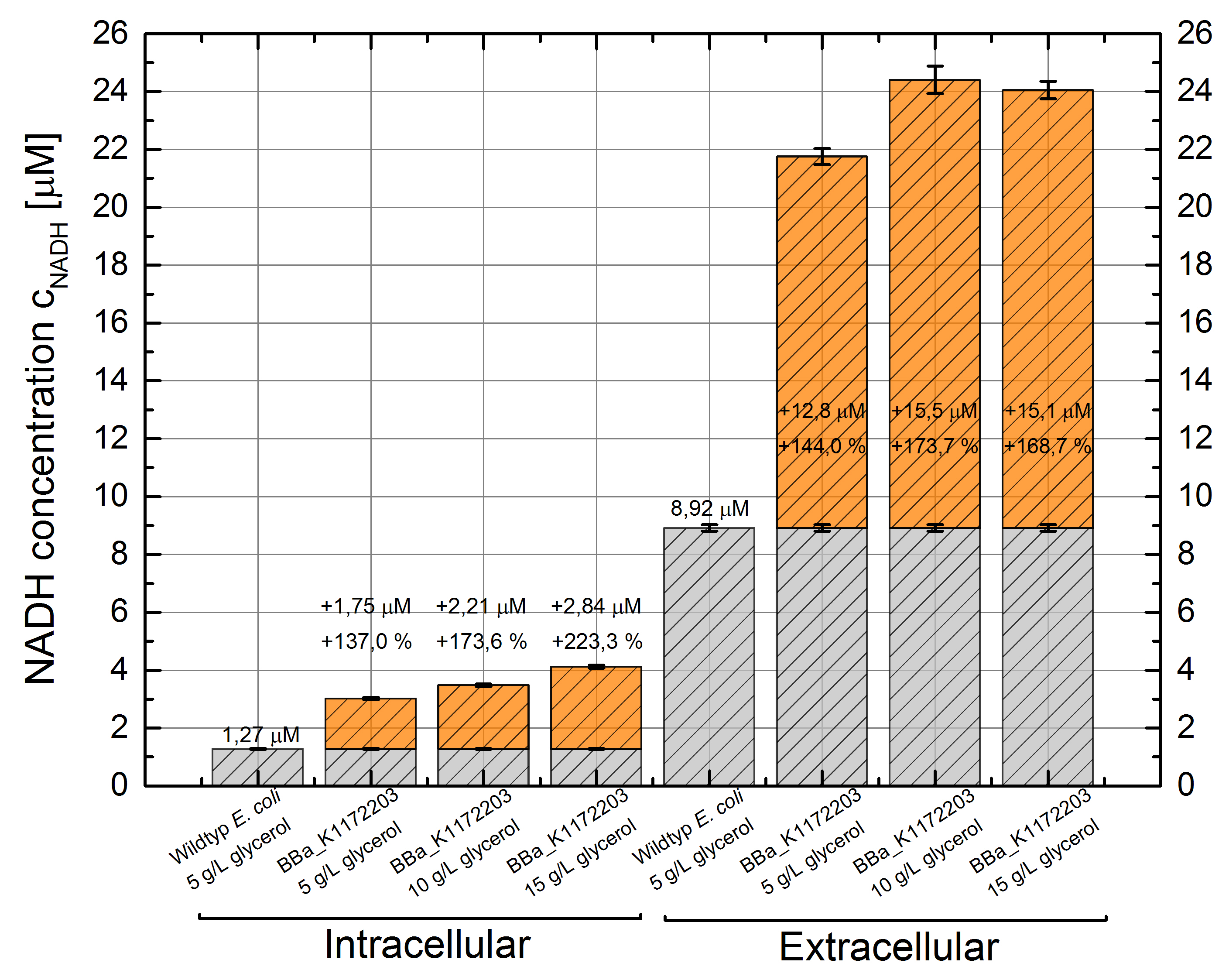
Figure 9: Column Chart with results of the NADH-Assay. Comparison between Escherichia coli KRX Wildtyp and Escherichia coli KRX with <bbpart>BBa_K1172203</bbpart> grown in M9-medium with different concentrations of glycerol. NADH concentration with standard deviation and concentration in comparison to the wild-type is shown for cell disruption (Intracellular) and supernatant (Extracellular) fraction.
Cytochromes
Biosafety
Porines
Contents |
 "
"