Team:Uppsala/zeaxanthin
From 2013.igem.org
| Line 94: | Line 94: | ||
<li><a href="https://2013.igem.org/Team:Uppsala/carotenoid-group">Carotenoid group</a></li> | <li><a href="https://2013.igem.org/Team:Uppsala/carotenoid-group">Carotenoid group</a></li> | ||
<li><a href="https://2013.igem.org/Team:Uppsala/chassi-group">Chassi group</a></li> | <li><a href="https://2013.igem.org/Team:Uppsala/chassi-group">Chassi group</a></li> | ||
| + | <li><a href="https://2013.igem.org/Team:Uppsala/advisors">Advisors</a></li> | ||
</ul></li> | </ul></li> | ||
| Line 105: | Line 106: | ||
</ul></li> | </ul></li> | ||
| - | <li><a href="https://2013.igem.org/Team:Uppsala/attribution" id="list_type4"><img class="nav-text" src="https://static.igem.org/mediawiki/2013/5/5d/Uppsala2013_Attributions.png"></a | + | <li><a href="https://2013.igem.org/Team:Uppsala/attribution" id="list_type4"><img class="nav-text" src="https://static.igem.org/mediawiki/2013/5/5d/Uppsala2013_Attributions.png"></a></li> |
| - | + | ||
| - | + | ||
| - | + | ||
<li><a href="https://2013.igem.org/Team:Uppsala/notebook" id="list_type3"><img class="nav-text" src="https://static.igem.org/mediawiki/2013/3/36/Uppsala2013_Notebook.png"></a> | <li><a href="https://2013.igem.org/Team:Uppsala/notebook" id="list_type3"><img class="nav-text" src="https://static.igem.org/mediawiki/2013/3/36/Uppsala2013_Notebook.png"></a> | ||
<ul> | <ul> | ||
<li><a href="https://2013.igem.org/Team:Uppsala/safety-form">Safety form</a></li> | <li><a href="https://2013.igem.org/Team:Uppsala/safety-form">Safety form</a></li> | ||
| + | <li><a href="https://2013.igem.org/Team:Uppsala/protocols">Protocols</a></li> | ||
</ul></li> | </ul></li> | ||
</ul> | </ul> | ||
| Line 183: | Line 182: | ||
<div id="bottom-pic"> | <div id="bottom-pic"> | ||
| - | + | ||
</div> | </div> | ||
Revision as of 22:26, 3 October 2013
Zeaxanthin

Zeaxanthin is a yellow carotenoid pigment, derived from the precursor ß-carotene through hydroxylation by the enzyme ”Beta-carotene hydroxylase”. Zeaxanthin acts as an antioxidant and can be found in for example peppers, yolk and maize.(1) According to studies zeaxanthin has positive effects on both undamaged and impaired vision and it may prevent age-related macular degeneration (AMD), an eye condition that could lead to blindness. (2) Furthermore studies have also indicated that zeaxanthin may have skin protective activities. (1,3)
Except for having beneficial properties the carotenoid zeaxanthin is one of the primary precursors for the production of the saffron metabolites picrocrocin, crocin and safranal. The production of saffron was inspired by the work of Washington team WashU iGEM 2012. Since the gene (CrtZ) that was used for the production of zeaxanthin was a eukaryotic version from Arabidopsis Thaliana, we decided to focus on finding a suitable gene to optimize the production of the above-mentioned metabolites. When searching for an appropriate gene we came in contact with Slovenia iGEM 2010 that had produced zeaxanthin in E.coli. The operon that was obtained contained genes that were originally from the bacteria Pantoea Anantis.
Methods:
For the characterization of zeaxanthin in E.coli DH5alpha we put together a standardized method for zeaxanthin extraction and measuring. Since zeaxanthin in its pure form is extremely expensive (~ 480 €/mg) we bought maize at our local supermarket and carried out liquid-liquid extractions using methanol and performed spectrophotometry measurements. We compared literature absorbance values of zeaxanthin to the peaks we obtained from our own measurements and continued our experiments until we believed to have an optimized extraction method.
 Figure 1. Maize bought at our local supermarket that was grinded and later used to make a standardized zeaxanthin extraction method.
Figure 1. Maize bought at our local supermarket that was grinded and later used to make a standardized zeaxanthin extraction method.
Figure 2. Extraction experiments carried out on grinded maize using methanol.
The operon acquired from Slovenia iGEM 2010 consists of the genes CrtE, CrtB, CrtI, CrtY and CrtZ assembled with the inducible promoter pBAD/AraC.
Since we would eventually like to produce zeaxanthin in Lactobacillus in yoghurt it would be preferable to have a constitutive promoter, such as the CP-promoters in our promoter library. Parallel to the characterization of zeaxanthin in E.coli we therefore attempted to remove the pBAD/AraC promoter from the zeaxanthin operon.

Results:
Spectrophotometry
Production of zeaxanthin in E.coli DH5alpha was detected trough spectrophotometry measurements performed after liquid-liquid extractions using methanol. The resulting absorbance curve was compared to the standardized curve. In both graphs you can find the two peaks corresponding to zeaxanthin.
 Figure 3. E.coli culture believed to have zeaxanthin production.
Figure 3. E.coli culture believed to have zeaxanthin production.
Figure 4. A liquid-liquid extraction was carried out on the E.coli culture containing the plasmid above. Methanol was used as an organic solvent.
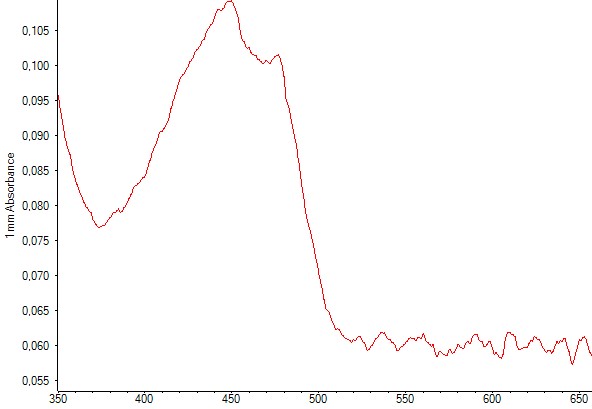

Figure 6. Spectrophotometry of an E.coli culture containing the plasmid with the zeaxanthin operon. The characteristic peaks of zeaxanthin are present, indicating that zeaxanthin was present in the sample.
Spectrophotometry measurements were also done on unmodified E.coli, as a negative control. These measurements did not result in the peaks that are characteristic for zeaxanthin.
 Figure 7. Spectrophotometry of unmodified E.coli did not result
Figure 7. Spectrophotometry of unmodified E.coli did not resultin the peaks characteristic for zeaxanthin.
 "
"









