
P-Coumaric Acid
Opening up the phenylpropanoid pathway to iGEM
The phenylpropanoid pathway metabolites, which starts with the aminoacids tyrosine or phenylalanine as precursors has many important health aspects. Tyrosine ammonia lyase produces p-coumaric acid from tyrosine. We have searched the parts registry of a working and characterized tyrosine ammonia lyase, but all earlier iGEM attempts showed no characterization of availability whatsoever. That is why we decided to make a fully working and characterized biobrick of tyrosine ammonia lyase. By using a new to iGEM bacterial version of tyrosine ammonia lyase, we will open up the diverse metabolic pathway of flavonoids, anthocyanins, and stilbenes, to name a few. [1] In this project, we will try to produce the antioxidants p-coumaric acid and resveratrol.
P-coumaric acid
P-coumaric acid is an antioxidant with many beneficial health aspects.It has been shown that it reduces the risk of one of our greatest modern western diseases, atherosclerosis.[2] It has also been shown to reduce the risk of stomach cancer. [3] That is why we think this is a very important ingredient in our probiotic bacteria.
|
 |
Methods
Tyrosine ammonia lyase (TAL) from rhodobacter sphaeroides was obtained from J.Conrado et al.[4]
The previous versions used in iGEM have all been from eukaryotic species. We biobricked TAL with the ribosome binding site B0034 and overhangs in a single pcr with primer overhangs. We also made a version with 6-HIS-tag for enzyme expression analysis. TAL was also mutagenized to remove illegal Not1 site. We verify all of our genetical constructs with sequencing.
We have expressed TAL in e-coli DH5alpha and E-coli nissle, a probiotic e-coli obtained from Trieste iGEM 2012. TAL will also be characterized in lactobacillus, by transforming the construct with our shuttle vector.
This construct can also be used to produce the precursor for our resveratrol.

Results
Summary
We managed to clone out and biobrick tyrosine ammonia lyase and verify the biobrick by sequencing. Also we did succeful characterization on this part, showing that it works as expected. We managed to express our enzyme and detect it in a western blot, and also detect our metabolite in both spectrophotometry and hplc. The biobrick was characterized in e-coli d5halpha and e-coli nissle. For detailed information about the characterisation methods, see the protocol section.
Biobrick
We succeded in the cloning and sequencing of our biobrick, Tyrosine ammonia lyase from
rhodobacter sphaeroides with the RBS B0034 that should work in various organisms, lactobacillus
and e-coli. Sequencing was done at GATC biotech and Uppsala Genome center using sanger
sequencing.
Tyrosine ammonia lyase with rbs:
BBa_K1033000
Western blot
We also succeeded in expressing the enzyme tyrosine ammonia-lyase (TAL) in e-coli.
To enable the detection of this protein by anti-his antibodies, 6-histidine tags was incorporated in the
sequence. This way we could detect our enzyme with anti-his antibodies.
We expressed our protein with a promotor working in both lactobacillus and e-coli. This way, we can
easily transfer TAL to lactobacillus later on.
The size of our protein was calculated using ProtParam
[5], 54.9 kDA.

1. Positive control
2. TAL with Cp8 promotor
3. TAL with J23110 promotor
4. Negative control
Figure 1:SDS-page and western blot. Expression of Tyrosine ammonia lyase with constitutive promotors. The negative control is empty, showing that there is no natural protein in e-coli with the same attributes.
Spectrophotometry
As the next step, we have made a spectrophotometric assay of our metabolite p-coumaric acid produced by e-coli. By using n-octanol and a two-phase extraction, we were able to extract our metabolite from the lb medium and bacteria. This way we could characterize our biobrick on spectrophotometer. The positive control we used had a final p-coumaric concentration of 500 µl. In our spectrophotometry we can see that we have a somewhat lower concentration after 48 hours of stationary phase incubation.
The absorbance spectra of extracts from bacterial cultures grown at stationary phase for 48 hours were collected and the concentration of p-coumaric acid was calculated from the absorbance spectra in an ordinary least squares manner with a
MATLAB-script. The concentrations of p-coumaric acid in the cultures were: 228 µM for pSB3K3-J23101-TAL, 158 µM for pSB3K3-J23110-TAL and 311 µM for pSB3K3-CP8-TAL (spectrum shown in figure 2 below).
c
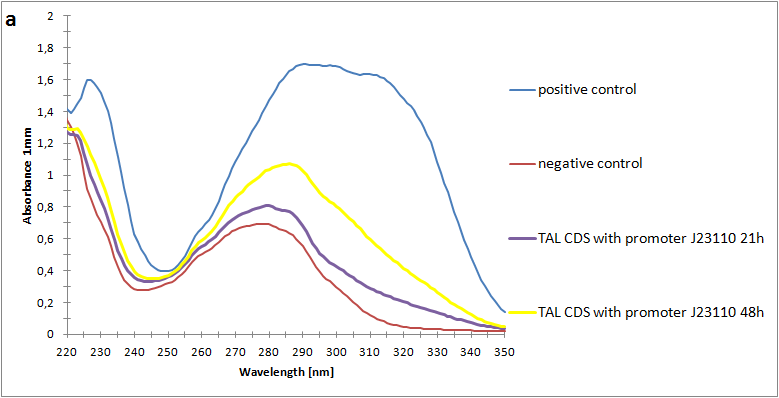
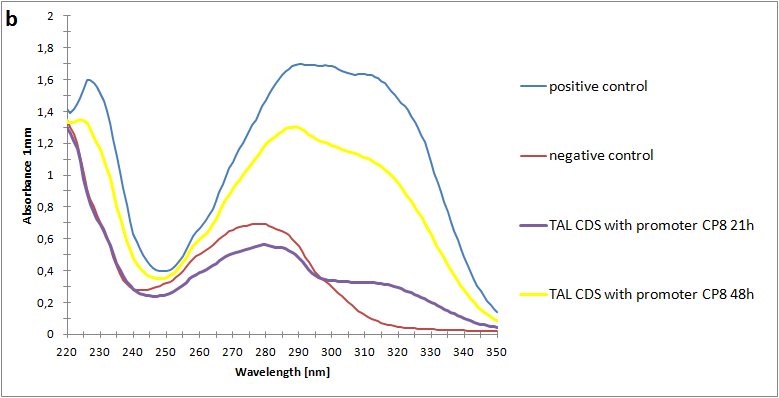

Figure 2:Absorbance spectra of extracts collected from bacterial cultures of strain pSB3K3-CP8-TAL. Samples were collected 21 h and 48 h after 30 °C incubation. Negative control is an extract from a strain with no TAL gene on the transformed plasmid. The positive control is an extract on a culture of the same strain as the negative control but with added p-coumaric acid to a concentration of 500 µM before extraction. The p-coumaric acid absorbance peak is around 305 nm.
High pressure liquid chromatography
We also saw in our hplc that we have succefull production of p-coumaric acid in e-coli. The next step will be to transfer the genes with our shuttle vector to express them in lactobacillus.)
 | Figure 3. Graph showing the HPLC result of a sample prepared from e coli expressing tyrosine ammonia lyase. Reverse phase HPLC with a C18 matrix was used. The peak for p-coumaric acid can be seen ~9 min, as shown by the standard sample below. |
 | Figure 4. Graph showing the HPLC result of a sample standard with p-coumaric acid |
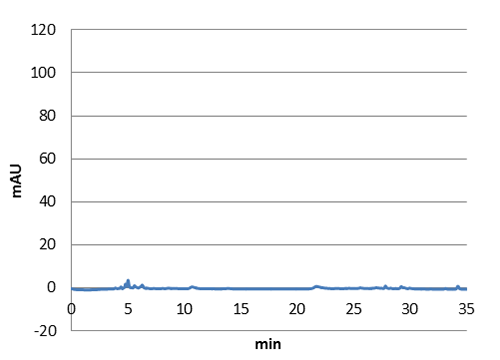 | Figure 5. E-coli culture injected to the hplc without our biobrick tyrosine ammonia lyase. Here we can see that there is originally no peak at 9 minutes. |
References
[1]Zhixion Xue et al, Identification and functional expression of tyrosine ammonia-lyase and its mutants from the photosynthetic bacterium rhodobacter sphaeroides, J Ind Microbiol Biotechnol (2007) 34:599-604
[2] Lun-Yi Zang et al, Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation, Am J Physiol Cell Physiol. 2000 Oct;279(4):C954-60.
[3]Lynnette R. Ferguson, Shuo-tun Zhu, Philip J. Harris, Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells, Issue, Molecular Nutrition & Food Research, Volume 49, Issue 6, pages 585–593, June 2005
[4]Robert J. Conrado et al, DNA guided assembly of biosynthetic pathways promotes improved catalytic effiency. Nucleic Acids Research , 2012, Vol 40 NO 4, 1879-1889
[5] http://web.expasy.org/protparam/
 "
"




















