Team:Bielefeld-Germany/Project/MFC
From 2013.igem.org
| Line 127: | Line 127: | ||
==Exogenous Mediators== | ==Exogenous Mediators== | ||
| - | + | <p align="justify"> | |
| - | + | Look here (LINK HIER XXX) for general information about redox mediator molecules. | |
| - | + | Redox molecules which are chemically synthesized can be added into the anode chamber of the fuel cell to act as mediators, transporting electrons from the bacteria to the fuel cells anode. Here, these chemicals are referred to as exogenous mediators.<br> | |
| + | <br> | ||
| + | To identify an exogenous mediators which can easily be reduced by <i>e. coli</i>, a simple experiment was conducted. A culture of <i>e. coli</i> KRX was distributed into 1.5 mL reaction tubes. Methylene blue and neutral red were added in varying concentrations. Since all mediators tested lose their color when reduced, a change in the color of the solution means, that the bacteria are able to reduce the respective compound. Incubation took place at 24°C for 24 hours. | ||
| + | </p> | ||
| + | <br> | ||
| + | [[File:IGEM_Bielefeld2013_exomediators.png|500px|center|thumb|'''Figure XXX:''' Reaction tubes with bacteria and different concentrations of methylene blue and neutral red after 24 hours of incubation.]] | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | In Fig XXX it is clearly visible that in some of the vessels where methylene blue was added, the lower half of the solution lost its colour. Oxygen is able to oxidize leucomethylene blue back to the molecules blue form, so a blue color near the liquids surface is to be expected. Based on these results, methylene blue was chosen as the preferred exogenous mediator for further experiments. | ||
| + | </p><br><br> | ||
| + | [[File:IGEM_Bielefeld2013_exomediators2.png|500px|center|thumb|'''Figure XXX:''' Lewis structures of the cationic form of methylene blue (right side) and the reduced form, called leucomethylene blue (left side).]] | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | When a methylene blue salt (like XXXWAS BENUTZEN WIR?) is dissolved in water, it dissociates into an anion and a new methylene blue cation (see fig. XXX). The molecules conjugated electron systems causes methylene blue solutions to appear in a dark blue color.<br> | ||
| + | The compound can be reduced, accepting an electron and a proton in the process (see figure xxx). This breaks up the conjugated electron system, which is why leucomethylene blue is colorless. The [http://employees.csbsju.edu/hjakubowski/classes/ch331/oxphos/standredpotentialtab.htm standard potential] of the methylene blue / leucomethylene blue reaction is +0.01 V. | ||
| + | </p> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
==Cultivation== | ==Cultivation== | ||
Revision as of 17:24, 4 October 2013
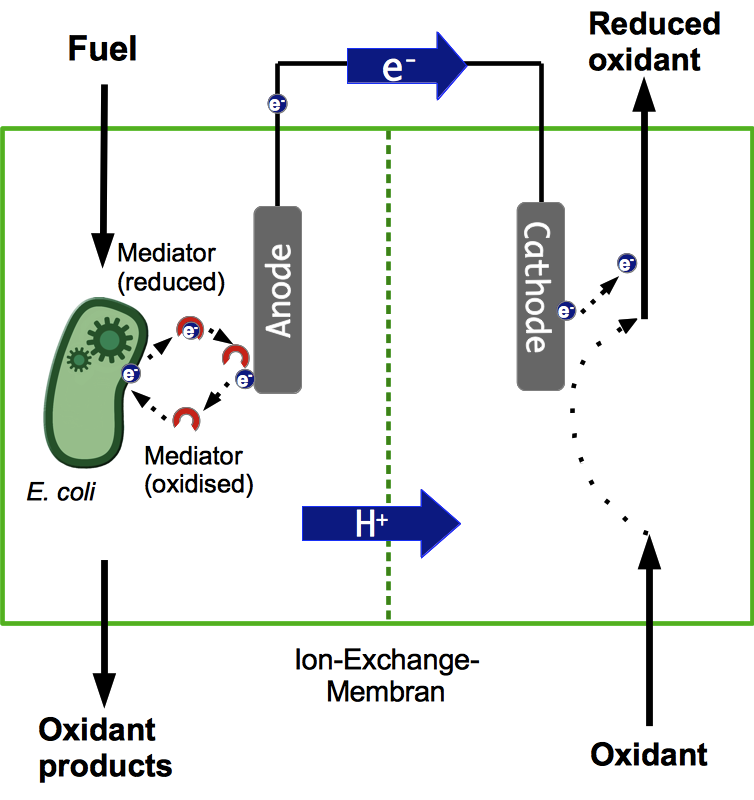
Microbial Fuel Cell
Basic Principle of a Microbial Fuel Cell
A microbial fuel cell (MFC) can be utilized for power generation through the conversion of organic substrates by microorganisms. The fuel cell generally consists of two separate units, the anode and cathode compartment. Both compartments are separated by a proton exchange membrane (PEM). Microorganisms acting as biocatalysts release electrons during metabolic reactions and transfer them to the anode of the fuel cell. The simultaneously generated protons are transferred through the PEM directly to the cathode compartment, while the electrons have to pass through an external load circuit. These electrons reduce an electron acceptor on the cathode side. Both anaerobic and aerobic electron acceptors may be used. Optimization of electron transfer is an important parameter for the effective use of an MFC. Generally, electrons can be transferred to the anode either by direct contact or through mediators.
Theory
Construction
Media and carbon sources
As explained previously (XXXLINKHIER), E. coli have to respire under aerobic conditions in order for the microbial fuel cell to work. This can prove to be problematic when using media such as LB, because in the absence of oxygen the bacteria might use the available carbon source for fermentation. To prevent this, the different strains which were tested in the fuel cell were cultivated in M9 minimal medium. After consulting about the fermentation problem with Dr. Falk Harnisch, we decided to supplement the M9 medium with different carbon sources to test how this affects bacterial growth under anaerobic conditions. The goal of this experiment was to find a carbon source which is well suited for aerobic respiration, but can’t be used for fermentation by the bacteria.
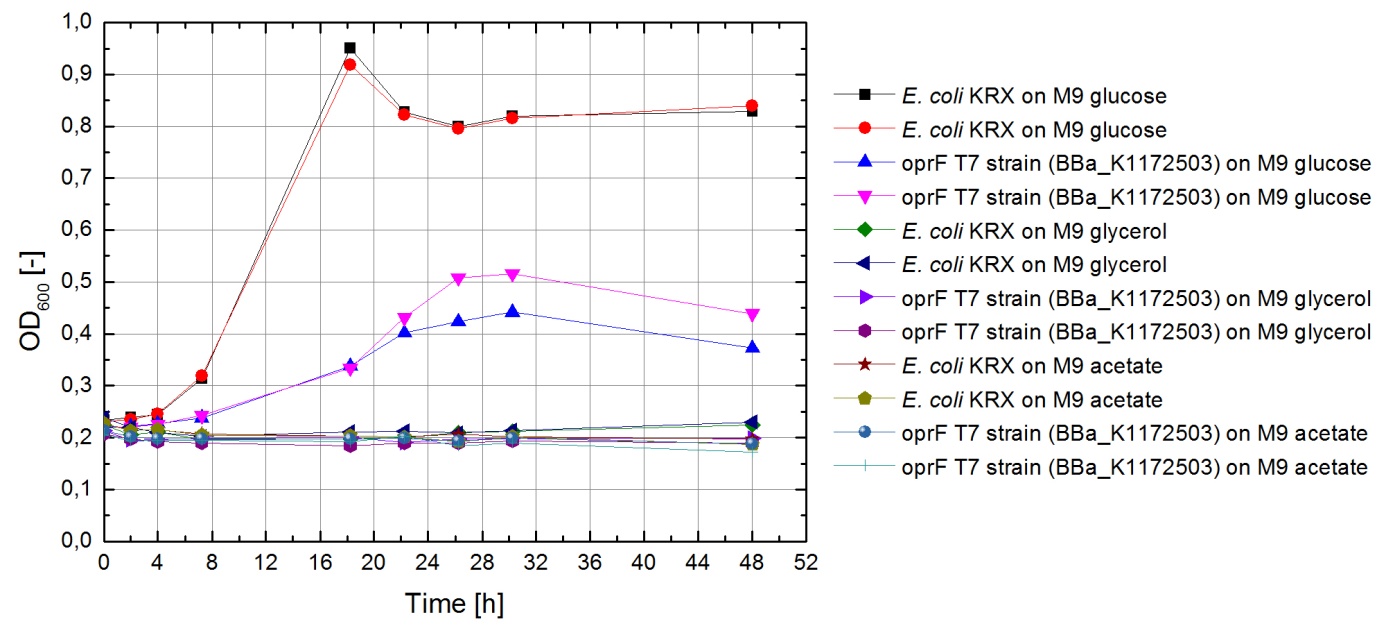
Tests were carried out with glucose, glycerol and acetate. The amount of substrate supplemented into the medium was adjusted, so that the amount of carbon atoms was the same for each culture. The concentrations were 5.0 g/L for glucose, 5.1 g/L for glycerol and 6.8 g/L for acetate. In all cultivation experiments, two biological replica of every strain and substrate combination were prepared and each sample was diluted and measured twice. Anaerobic cultivations were conducted in 30 mL test tubes with rubber lids. Samples were taken by piercing the lid with a syringe. For aerobic cultivations, shaker flasks were used. The strains tested were the KRX wild-type and the KRX oprF T7 ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1172502 BBa_K1172502]) strain. The latter overexpresses large porin-proteins when induced with rhamnose. Induction was carried out with XXX mL of a XXX g/L rhamnose solution 1 hour after inoculation. All cultures were inoculated with an optical density of 0.22. Temperature was kept at 37°C during cultivation.
Figure 1 shows that, under anaerobic conditions, both strains grow well on glucose but seem to be unable to use acetate or glycerol efficiently.
To ascertain whether glucose, glycerol or acetate make for good substrates under aerobic conditions, eight additional cultures were inoculated with both strains. For these cultures, only an end-point determination was carried out. The results can be seen in figure 2. They indicate that acetate is, as expected, not a suitable substrate. Growth is best with glucose as a substrate, but glycerol yields satisfactory results as well.
The data acquired points to glycerol being the most suitable substrate. To make sure glycerol can also be used for respiration with terminal electron acceptors other then oxygen, bacteria were cultivated under anaerobic conditions in M9 medium containing glycerol as a carbon source and potassium nitrate as a soluble electron acceptor.
The results of this experiment are shown in figure 3. Cultures which were supplemented with KNO3 grow significantly faster than those that weren’t, indicating that potassium nitrate is indeed used as a terminal electron acceptor for anaerobic respiration.
Based on these results, M9 medium with 5.1 g of glycerol per liter is used for all future experiments with the fuel cell.
Exogenous Mediators
Look here (LINK HIER XXX) for general information about redox mediator molecules.
Redox molecules which are chemically synthesized can be added into the anode chamber of the fuel cell to act as mediators, transporting electrons from the bacteria to the fuel cells anode. Here, these chemicals are referred to as exogenous mediators.
To identify an exogenous mediators which can easily be reduced by e. coli, a simple experiment was conducted. A culture of e. coli KRX was distributed into 1.5 mL reaction tubes. Methylene blue and neutral red were added in varying concentrations. Since all mediators tested lose their color when reduced, a change in the color of the solution means, that the bacteria are able to reduce the respective compound. Incubation took place at 24°C for 24 hours.
In Fig XXX it is clearly visible that in some of the vessels where methylene blue was added, the lower half of the solution lost its colour. Oxygen is able to oxidize leucomethylene blue back to the molecules blue form, so a blue color near the liquids surface is to be expected. Based on these results, methylene blue was chosen as the preferred exogenous mediator for further experiments.
When a methylene blue salt (like XXXWAS BENUTZEN WIR?) is dissolved in water, it dissociates into an anion and a new methylene blue cation (see fig. XXX). The molecules conjugated electron systems causes methylene blue solutions to appear in a dark blue color.
The compound can be reduced, accepting an electron and a proton in the process (see figure xxx). This breaks up the conjugated electron system, which is why leucomethylene blue is colorless. The [http://employees.csbsju.edu/hjakubowski/classes/ch331/oxphos/standredpotentialtab.htm standard potential] of the methylene blue / leucomethylene blue reaction is +0.01 V.
Cultivation
Results
References
 "
"