Team:Bielefeld-Germany/Biosafety/Biosafety System S
From 2013.igem.org
| Line 69: | Line 69: | ||
<p align="justify"> | <p align="justify"> | ||
| - | + | The araC gene is naturally found in ''E. coli'' and is coding for the 32 kDa araC protein, which regulates the expression of the genes required for the uptake and catabolism of the pentose L-arabinose. The genes for the catabolism of arabinose are under the control of the arabinose promoter pBAD, which is both positively and negatively regulated by the araC protein. So naturally in the presence of L-arabinose the expression of this genes is activated, while it is repressed in its absence. Apart the araC protein regulates his own transcription under the control of the so called pC promoter. Compared to the pBAD promoter the pC promoter as well as the araC gene is thereby transcripted in the opposite direction ([https://2013.igem.org/Team:Bielefeld-Germany/Biosafety/Biosafety_System_S#References Schleif, 2010]). | |
</p> | </p> | ||
| + | |||
Revision as of 01:55, 5 October 2013
Biosafety System AraCtive
Overview
The araC gene is naturally found in E. coli and is coding for the 32 kDa araC protein, which regulates the expression of the genes required for the uptake and catabolism of the pentose L-arabinose. The genes for the catabolism of arabinose are under the control of the arabinose promoter pBAD, which is both positively and negatively regulated by the araC protein. So naturally in the presence of L-arabinose the expression of this genes is activated, while it is repressed in its absence. Apart the araC protein regulates his own transcription under the control of the so called pC promoter. Compared to the pBAD promoter the pC promoter as well as the araC gene is thereby transcripted in the opposite direction (Schleif, 2010).
Genetic Approach
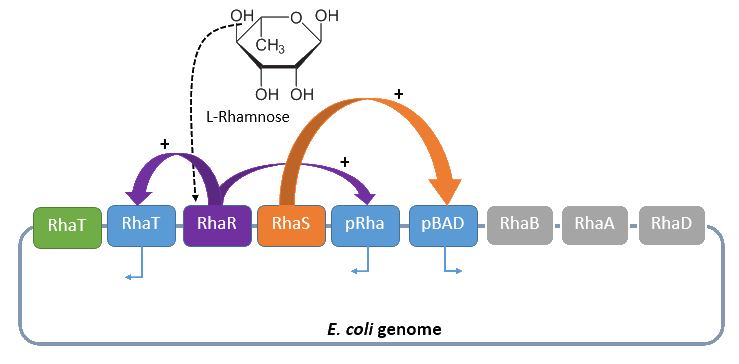
Rhamnose promoter rhaBAD
The promoter rhaBAD regulates naturally the catabolism of the hexose L-rhamnose. The advantage of the operon is its positively regulation. All in all the regulon consists of the promoter pRhaT, who regulates the expression of the protein RhaT for the uptake of L-Rhamnose, the operons rhaSR and rhaBAD.
The operon rhaSR regulates the genes RhaS and RhaR, whose translated proteins are responsible for the positive activation of the L-Rhamnose catabolism, while the operon rhaBAD regulates the genes for the direct catabolism of L-Rhamnose.
When L-rhamnose is present, it acts as an inducer by binding to the regulatory protein RhaR. RhaR regulates his own expression and the expression of the regulatory gene RhaS by inhibiting or activating, in the presence of L-Rhamnose, the operon RhaSR. Normally the expression level is modest, but it can be enhanced by a higher level of intracellular cAMP, which increases in the absence of glucose. So in the presence of L-Rhamnose and a high concentration of intracellular cAMP the activor protein RhaR is expressed on higher level, resulting in an activation of the promoter pRhaT for an efficient L-rhamnose uptake and an activation of the operon rhaBAD. The L-Rhamnose is than broken down into dihydroxyacetone phosphate and lactate aldehyde by the enzymes of the operon rhaBAD (Wickstrum et al., 2005).
Dihydroxyacetone phosphate can be metabolized in the glycolysis pathway, while the lactate aldehyde is oxized to lactate under aerobic conditions and reduced to L-1,2,-propandiol under anaerobic conditions.
This degradation of L-Rhamnose can be separated in three steps. In the first step the L-Rhamnose is turned into L-Rhamnulose by an isomerase (gene RhaA). The catabolism continues by the a kinase (gene RhaB), who phosphorylates the L-Rhamnulose to L-Rhamnulose-1-phosphate. This is finally hydrolyzed by Aldolase (gene RhaD) to dihydroxyacetone phosphate and lactate aldehyde (Baldoma et al., 1988).
Four our Safety-System the rhamnose promoter rhaBAD is used to control the expression of the repressor araC and the essential Alanine-Racemase, because this promoter has even a lower basal transcription then the arabinose promoter pBAD. This is needed to tightly repress the expression of the Alanine-Racemase (alr) and take so advantage of the double-kill switch. So although the rhamnose promoter rhaBAD is characterized by a very low basal transcription it can not be used for the control of the toxic Barnase, because the regulation is mainly organized by an activation and not an active repression, but this is optimal for the first containing the Alanine-Racemase. So in conclusion this promoter is the best choice for the first part of the Biosafety-System, as it is tightly repressed in the absence of L-Rhamnose, but activated in its presence.
Repressor araC
The araC gene is naturally found in E. coli and is coding for the 32 kDa araC protein, which regulates the expression of the genes required for the uptake and catabolism of the pentose L-arabinose. The genes for the catabolism of arabinose are under the control of the arabinose promoter pBAD, which is both positively and negatively regulated by the araC protein. So naturally in the presence of L-arabinose the expression of this genes is activated, while it is repressed in its absence. Apart the araC protein regulates his own transcription under the control of the so called pC promoter. Compared to the pBAD promoter the pC promoter as well as the araC gene is thereby transcripted in the opposite direction (Schleif, 2010).
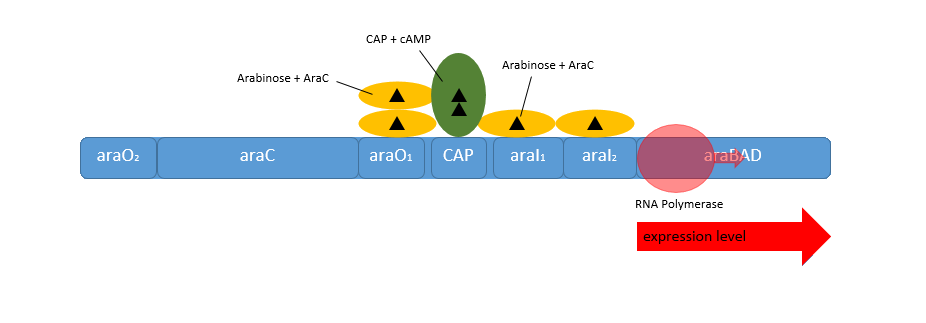
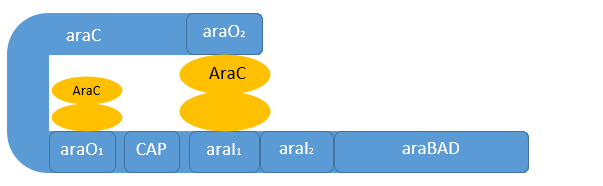
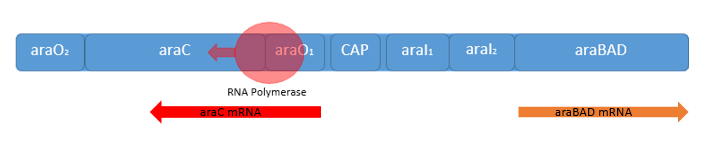
Naturally in the absence of L-arabinose the araC protein is found in its repressor conformation, binding to the O2-region and I1-region. The binding of two araC proteins to this domains leads to a protein-protein-interaction of araC forming a dimer. This araC is responsible for the tight repression of the pC and the pBAD promoter by forming a DNA-loop to inhibit the initiation of the RNA-polymerase (see figure above).
When L-arabinose is present in the media the operon changes to the active form. Following this conditions the araC protein functions as activator by binding to the I1- and I2-region of the operator region. This causes transcription of the genes behind the pBAD and the pC promoter. This could even be enhanced by a intracellular level of cAMP. In the absence of glucose the cell reaches a high level of glucose as a signal molecule. CAMP binds as the typically CRP-cAMP complex binds in front of the araC activator and increases the binding affinity of the RNA-polymerase, so that the genes behind the pBAD promoter are expressed on high level. Beside the pBAD promoter the araC protein regulates also the genes araE and the operon consisting the genes araFGH, who are necessary for an efficient uptake of L-arabinose (Cass, 1988).
The auto-regulation of the araC gene itself works both in the presence and absence of L-arabinose. It presence the DNA-loop which prevents also the transcription of the pBAD promoter inhibit also the transcription of araC. When L-arabinose is present and the araC concentration is high enough, its auto-regulates his own transcription by dimerization between the araO1- and the araO2-region. This leads to an other DNA-loop, inhibiting only the transcription of the genes behind the pC promoter (Hamilton, 1988).
For our Biosafety-System we decided to use the arabinose pBAD promoter, because this promoter is really tight regulated by araC, and does not even show a basal transcription in the complete absence of araC. Additional this promoter is repressed by glucose and activated on basal transcription in the presence of cAMP.
Alanine Racemase
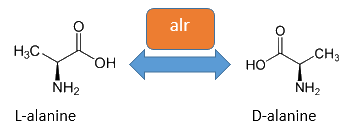
The alanine-racemase alr (EC 5.1.1.1) from the gram-negative enteric bacteria Escherichia coli is a racemase, which catalyses the reversible reaction from L-alanine into the enantiomer D-alanine. For this reaction the cofactor pyridoxal-5'-phosphate (PLP) is typically needed. The constitutive alanine-racemase (alr) is naturally responsible for the accumulation of D-Alanin, which is an essential component of the bacterial cell wall, because it is used for the crosslinkage of the peptidoglykan (Walsh, 1989).
The use of D-Alanine instead of a typically L-amino acids prevents the cleavage by peptdidases, but a lack of D-Alanine leeds to a bacteriostatic characteristic. So in the absence of D‑Alanine dividing cells will lyse rapidly. This approach is used by our Biosafety-Strain, a D-alanine auxotrophic mutant (K-12 ∆alr ∆dadX). The Safety-Strain grows only with a plasmid containing the Alanine-Racemase (<bbpart>BBa_K1172901</bbpart>) for the complementation of the D-alanine auxotrophic. Because the Alanine-Racemase is therefore essential for bacterial cell division, this approach guarantees a high plasmid stability, which is extremely important when the plasmid contains a toxic gene like the Barnase. In addition this construction provides the possibility of a double kill-switch system. Because if the expression of the Alanine-Racemase is repressed and there is no D-Alanine-Supplementation in the media, the cells would not increase.
Terminator
Terminator are essential for the end of an operon. In procaryot exists rho-depending and independing terminator. Rho-independing terminators are characterized by an stem-loop, which is caused by special sequence. In general the terminator-region can be divided into four regions. Starting with a GC-rich region, which performs the stem and followed by the loop-region. The third region is made up from the opposite part of the stem, so that this region concerns also GC-rich portion. After that the terminator ends by an poly uracil region, which destabilizes the binding of the RNA-polymerase. The stem-loop of the terminator causes a distinction of the DNA and the translated RNA, so that the binding of the RNA-polymerase is canceld and the transcription ends after the stem-loop (Carafa et al., 1990).
For our Safety-System the terminator is necessary to avoid that the expression of the genes under the control of the Rhamnose promoter pRHA, like the Repressor araC and the Alanine-Racemase (alr), are transcripted but not the genes of the Arabinose promoter pBAD, which contains the toxic Barnase and would lead to cell death.
pBAD promoter
The arabinose pBAD promoter controls the expression of the genes, who are necessary for the catabolism of the pentose arabinose. The expression is regulated by the activator and repressor araC. The genes of this operon convert the pentose to Xylose, which can be catalyzed into Fructose-1,6-diphosphate and becomes so part of the glycolyses. The conversion of the L-arabinose is realized in three steps. First of all the Isomerase (gene A) catalyses the reaction from L-arabinose into the isomer L-ribulose. In the second step the Ribulose-kinase (gene B) phosphorylates the L-ribulose to L-ribulose-monophosphat. For this reaction is also ATP needed. At least the L-ribulose-monophosphate is converted to D-Xylose-5-phosphat by the L-ribulose-5-phosphate-epimerase (gene D, Schleif, 2010).
For our Safety-System we used the arabinose promoter pBAD for the regulation of the toxic gene product Barnase. As this promoter has very low basal transcription and as the expression of the genes behind this promoter is strict depending on the presence of araC for activating the transcription, it is possible that this promoter controls a toxic gene product without causing cell death.
Barnase
The Barnase (EC 3.1.27) is an 12 kDa extracellular microbial ribonuclease, which is naturally found in the gram-positive soil bacteria Bacillus amyloliquefaciens and consist a single chain of 110 amino acids. The Barnase (RNase Ba) catalyses the cleavage of single stranded RNA, where the hydrolysis of the dinucleotides has the highest affinity to the structure GpN. In the first step of the RNA-degradation a cyclic intermediate is formed by transesterification and afterwards this intermediate is hydrolysed yielding in a 3'-nucleotide (Mossakowska et al., 1989).
In Bacillus amyloliquefaciens the activity ot the Barnase (RNase Ba) is inhibited intracelluar by the Inhibitor called barstar. Barstar consists only about 89 amino acids and binds with a high affinity to the toxic Barnase. This prevents the cleavage of the intracellular RNA in the host organism (Paddon et al., 1989). Therefore the Barnase acts naturally only outside the cell and is translocated under natural conditions. For the Biosafety-System we tried to modified this aspect by cloning only the sequence responsible for the cleavage of the RNA, but not the part of the native Barnase, which is essential for the extracellular transport.
As shown in the graphic below, the transcription of the DNA, which encodes the Barnase produces a 474 nt RNA. The translation of the RNA of this ribonuclease starts about 25 nucleotides downstream from the transcription start and can be divided into two parts. The first part (colored in orange) is translated into a signal peptide at the N-Terminus of the Barnase. This part is responsible for the extracellular translocation of the RNase Ba, while the peptide sequence for the active Barnase starts 142 nucleotides downstream from the transcription start (colored in red).
For the Biosafety-System we used only the coding sequence (<bbpart>BBa_K1172904</bbpart>) of the Barnase itself to prevent the extracellular translocation of the toxic gene product. This leads to a rapid cell death if the expression of the Barnase isn't repressed by the repressor of the Biosafety-System.
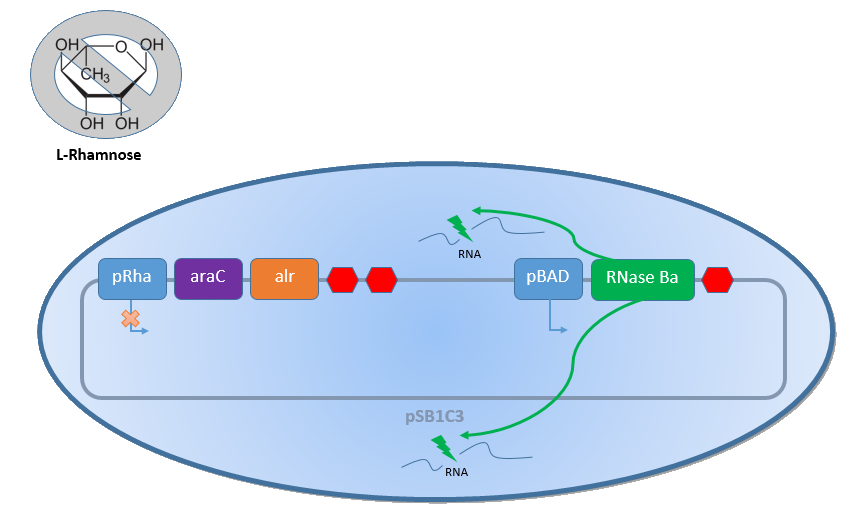
Biosafety-System araCtive
Together with the Biosafety-Strain K-12 ∆alr ∆dadX the Biosafety-System araCtive takes advantage of this genes by combine them to a powerful device, which allows to control the bacterial cell division. The control of the bacterial growth is thereby active and passive possible. Active by inducing the pBAD promoter with L-arabinose and passive by the induction of L-Rhamnose. The passive control makes it possible to control the bacterial cell division in an defined environment, like the MFC by adding continously L-Rhamnose to the media. As shown in the figure below, this leads to an expression of the essential Alanine-Racemase (alr) and the araC repressor, so that the expression of the RNase Ba is repressed.
When the bacteria exit the defined environment of the MFC or L-Rhamnose is not added any more to the media, the expression of the Alanine-Racemase (alr) and the araC repressor decreases, so that the expression of the toxic RNase Ba (Barnase) is not inhibted so strong any more. The cleavage of the intracellular RNA by the Barnase and ideally also the lack of synthesized D-alanine, caused by the repressed Alanine-Racemase inhibits the cell division and makes sure that the bacteria can only grow in the defined area.
Results
Characterization of the arabinose promoter pBAD
First of all the bacterial growth under the pressure of the unrepressed pBAD promoter was investigated on different carbon source. Therefore the cultivation on M9 minimal media with Glucose or Glycerol was characterized. To identify the transcription rate of the unrepressed arabionse promoter pBAD the expression of the green fluorescence protein GFP <bbpart>BBa_E0040</bbpart> behind the pBAD promoter was used by me.
As shown in figure 12 below, the bacteria adapted better on glucose then on Glycerol. As glucose is the more powerful energy source, because it posses more carbon atoms than glycerol these result was expected before. So more interesting are the fluorescence measurement shown in the figure 13. As it can be seen both the wild type K-12 as the Biosafety-Strain K-12 ∆alr ∆dadX ∆araC show about the same fluorescence on glucose (blue and black curve) but differ on glycerin. This can be explained by the fact that glucose itself also represses the arabinose promoter, while glycerol does not. In the presence of glucose the intracellular concentration of cAMP is low to repress the inefficient catabolism of arabinose, so that the glucose is catabolized first by the bacteria resulting in an optimal growth. In the absence of glucose increases, which enhances the transcription of the most operons, who regulate the enzymes for the catabolism of an alternative carbon source. Therefore the expression of GFP under the control of the pBAD promoter decreases on glycerol. Another different can be seen between the wild type (orange curve) and the Biosafety-Strain K-12 ∆alr ∆dadX ∆araC (red curve). The Biosafety-Strain shows lower expression then the wild type, but has according to figure 12 about the same growth rate. This is caused by the fact that the araC gene in the Biosafety-Strain was deleted an as the araC gene functions not only as are repressor but also as an activator the transcription rate decreases.
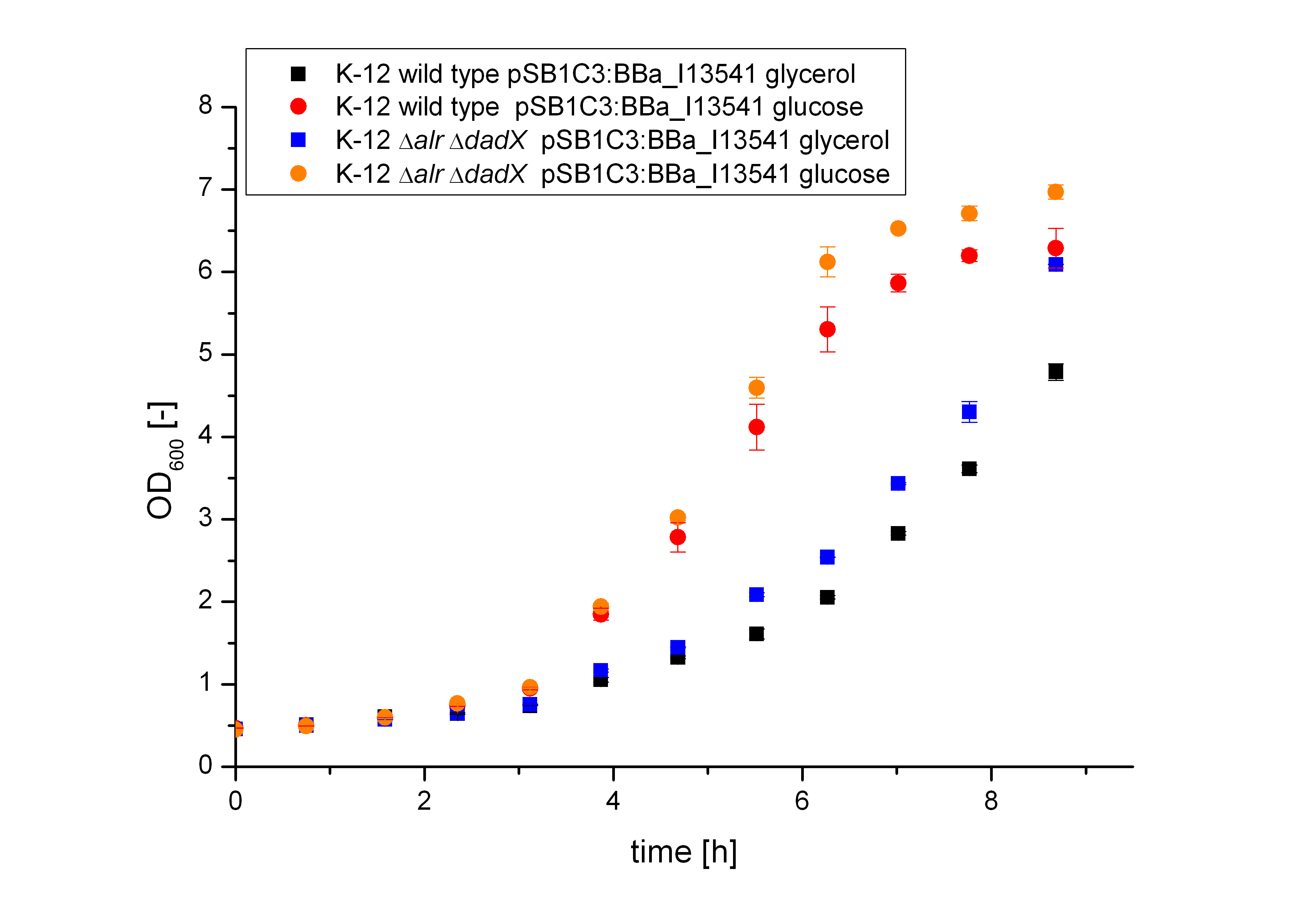
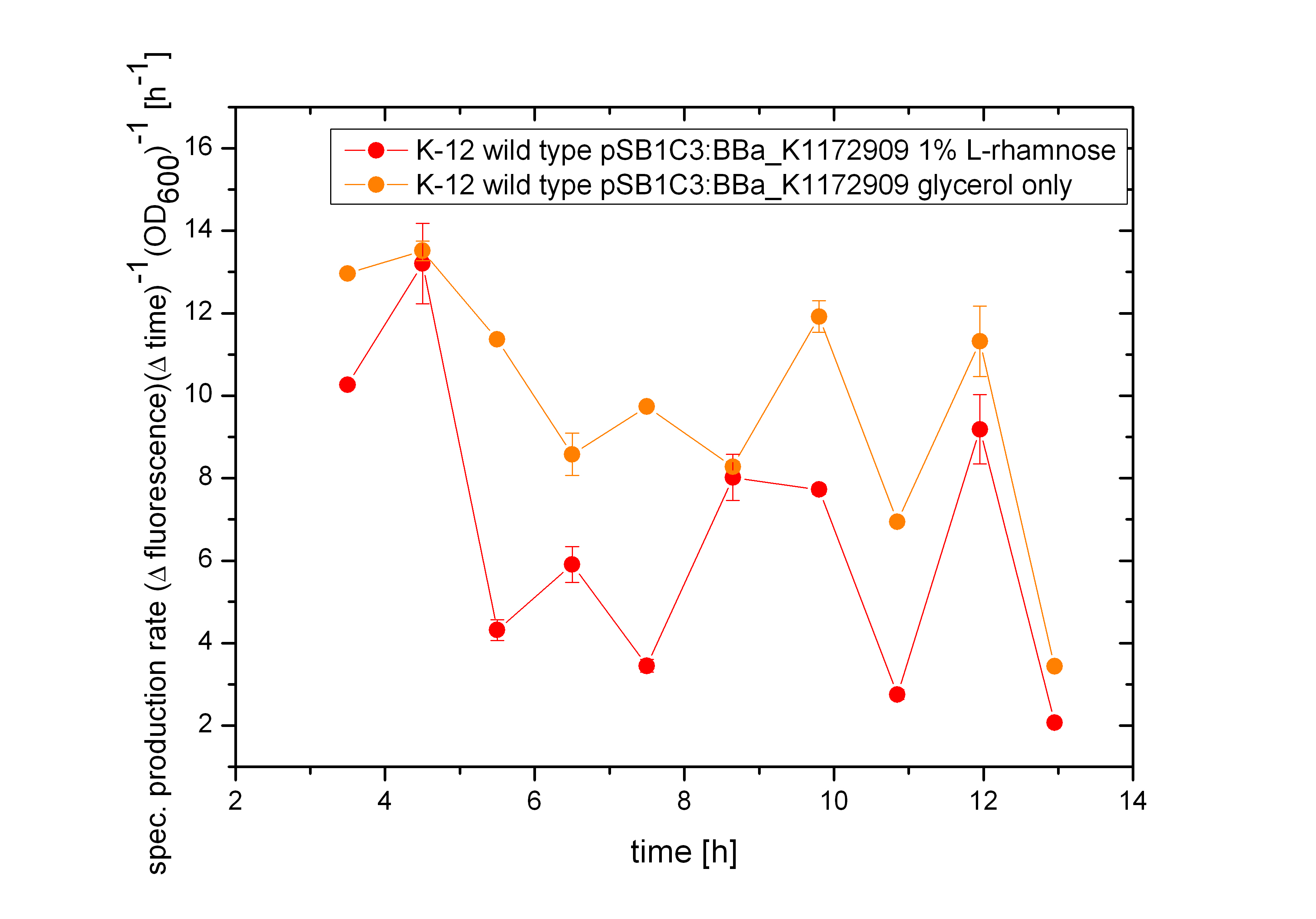
The effect that glucose represses the transcription of the pBAD promoter becomes more clear by the specific production rate, as shown in figure 14. The specific production rate was thereby calculated via equation (1) :
GLEICHUNG s p rate
So it can be seen that the building of GFP differs extremly between the cultivation on glucose and the cultivation of glycerol. While the production rate by using glucose as carbon source is nearly constant very low, it is thereby high in the cultivation with glycerol. As the specific production rate was calculated between every single measurement point the curve is not smoothed and so the fluctuations have to be ignored, as they do not stand for are real fluctuations in the transcription in the expression of GFP. They are caused by the growth curve and the fluorescence curve. And as they are not ideal there exists the fluctuations. But this graph shows clearly the difference between the two carbon sources.
Characterization of the Biosafety-System araCtive
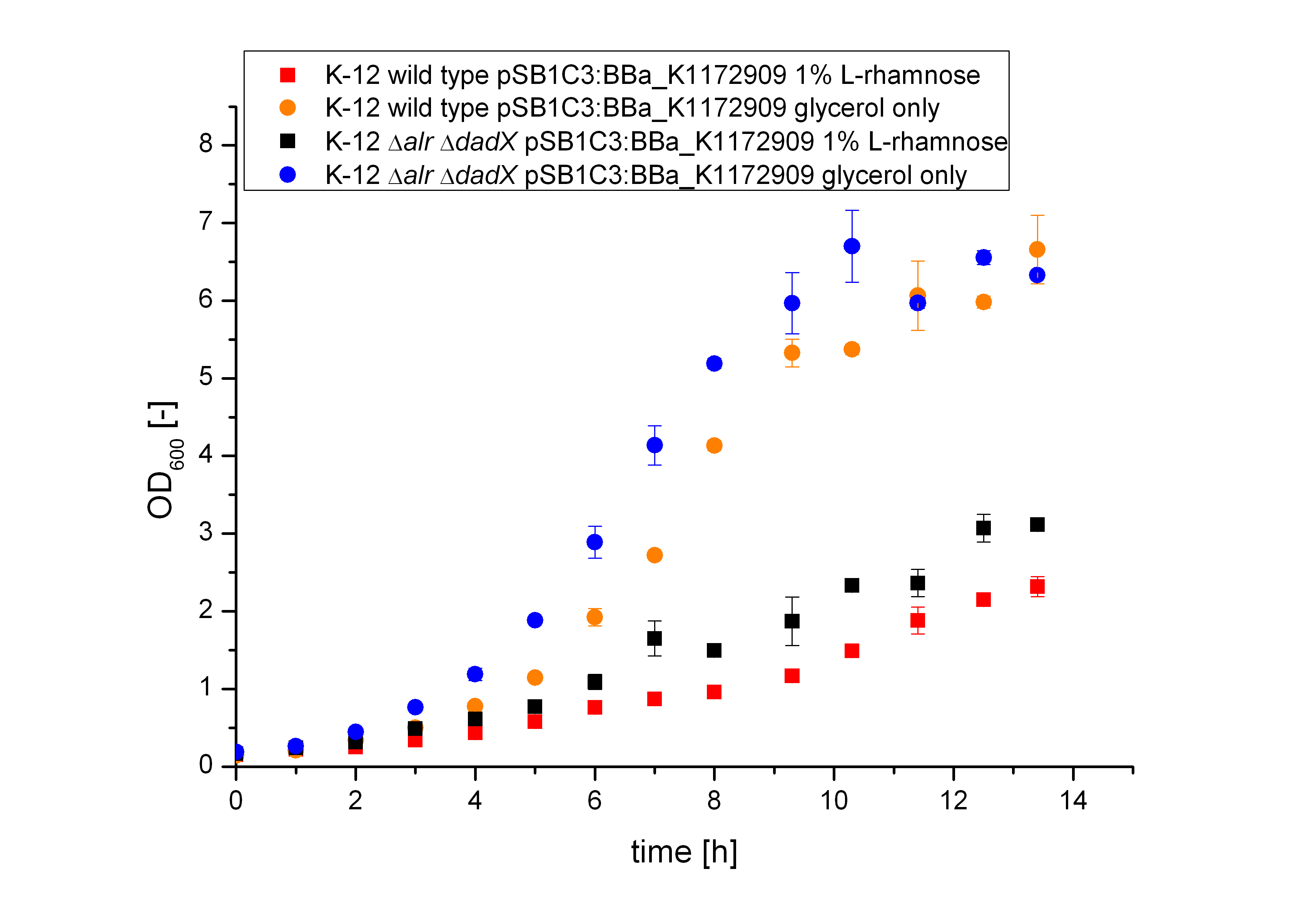
The Biosafety-System araCtive was characterized on M9 minimal medium with glycerol as carbon source. As for the characterization of the pure arabinose promoter pBAD above, the bacterial growth and the fluorescence of GFP <bbpart>BBa-E0040</bbpart> was measured. Therefore the wild type and the Biosafety-Strain E. coli K-12 ∆alr ∆dadX were cultivated once with the induction of 1% L-Rhamnose and once only on glycerol.
First of all it is obviously shown in figure 15 that the growth of the bacteria, who are induced with 1 % L-Rhamnose (blue and black curve) is significant slower than on pure glycerol (orange and red curve). This is attributed to the high metabolic pressure of the induced bacteria. The expression of the repressor araC and the Alanine-Racemase (alr) simultaneously causes a high outlay of the cells so that they grow slower then the uninduced cells, who expresses only GFP. Additional the arabinose promoter pBAD is tightly regulated, so that the expression even with a small amount of the repressor AraC is not that high and therefore not as stressful.
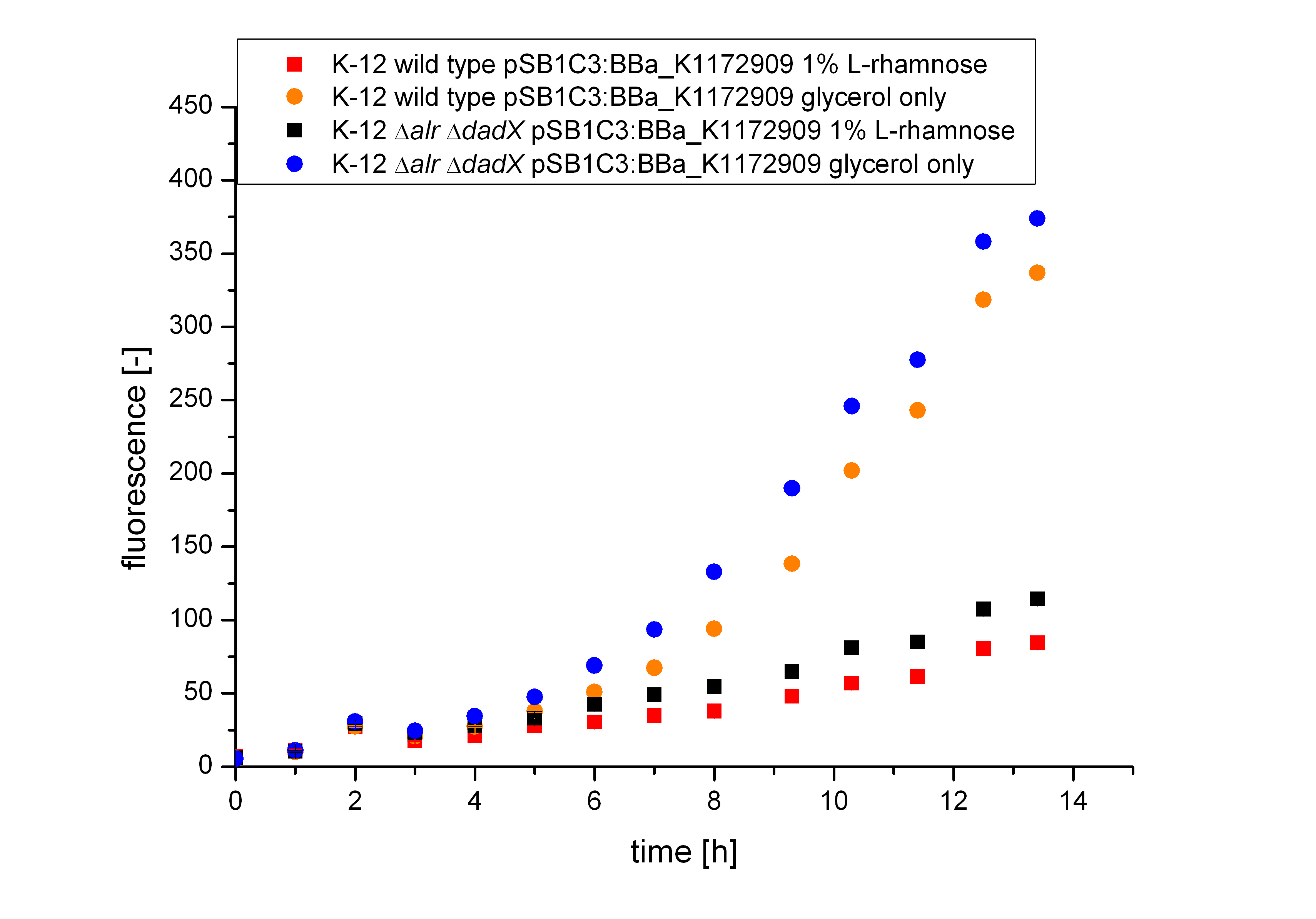
Comparing the bacterial growth with the fluorescence in figure 16 it can be seen that the fluorescence seems to follow the same figure than the bacterial growth. The uninduced cells shows approximately an exponantial rise of fluorescence, while the fluorescence of the induced bacteria increases only slow.
From the figure above it can not be seen if the expression of the repressor araC does effect the transcription of GFP or not. The slower growth of the bacteria is a first indication that the repressor araC and the Alanine-Racemase are highly expressed, but as the growth of the bacteria shows nearly the same figure than the fluorescence it could be possible that the repressor does not effect the expression level of GFP under the control of the arabinose promoter pBAD. That the Biosafety-System works as aspected by repressing the expression of GFP in the presence of L-Rhamnose can be seen from figure 18 below. The calculated specific production rate (equation 1) differs clearly, so that the production of GFP in the presence of L-Rhamnose is always lower than in its absence.
As the specific production rate was calculated between every single measurement point the curve is not smoothed and so the fluctuations have to be ignored, as they do not stand for are real fluctuations in the transcription in the expression of GFP. They are caused by the growth curve and the fluorescence curve. And this measured curves are not ideal the calculation of the specific production rate causes the fluctuations. But it can be seen very clear that the production of GFP differ an is much lower, when the bacteria are induced with 1% L-Rhamnose. So the Biosafety-System araCtive works.
Conclusion of the Results
References
- Baldoma L and Aguilar J (1988) Metabolism of L-Fucose and L-Rhamnose in Escherichia coli: Aerobic-Anaerobic Regulation of L-Lactaldehyde Dissimilation [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC210658/pdf/jbacter00179-0434.pdf|Journal of Bacteriology 170: 416 - 421.].
- Carafa, Yves d'Aubenton Brody, Edward and Claude (1990) Thermest Prediction of Rho-independent Escherichia coli Transcription Terminators - A Statistical Analysis of their RNA Stem-Loop Structures [http://ac.els-cdn.com/S0022283699800059/1-s2.0-S0022283699800059-main.pdf?_tid=ede07e2a-2a92-11e3-b889-00000aab0f6c&acdnat=1380629809_2d1a59e395fc69c8608ab8b5aea842f7|Journal of molecular biology 216: 835 - 858].
- Cass, Laura G. and Wilcoy, Gary (1988) Novel Activation of araC Expression and a DNA Site Required for araC Autoregulation in Escherichia coli B/r [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC211425/pdf/jbacter00187-0394.pdf|Journal of Bacteriology 170: 4174 - 4180].
- Hamilton, Eileen P. and Lee, Nancy (1988) Three binding sites for AraC protein are required for autoregulation of araC in Escherichia coli [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC279856/pdf/pnas00258-0029.pdf|Proc Natl Acad Sci U S A 85: 1749 - 53].
- Mossakowska, Danuta E. Nyberg, Kerstin and Fersht, Alan R. (1989) Kinetic Characterization of the Recombinant Ribonuclease from Bacillus amyloliquefaciens (Barnase) and Investigation of Key Residues in Catalysis by Site-Directed Mutagenesis [http://pubs.acs.org/doi/pdf/10.1021/bi00435a033|Biochemistry 28: 3843 - 3850.].
- Paddon, C. J. Vasantha, N. and Hartley, R. W. (1989) Translation and Processing of Bacillus amyloliquefaciens Extracellular Rnase [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC209718/pdf/jbacter00168-0575.pdf|Journal of Bacteriology 171: 1185 - 1187.].
- Schleif, Robert (2010) AraC protein, regulation of the L-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action [http://gene.bio.jhu.edu/Ourspdf/127.pdf|FEMS microbial reviews 34: 779 - 796.].
- Voss, Carsten Lindau, Dennis and Flaschel, Erwin (2006) Production of Recombinant RNase Ba and Its Application in Downstream Processing of Plasmid DNA for Pharmaceutical Use [http://onlinelibrary.wiley.com/doi/10.1021/bp050417e/pdf|Biotechnology Progress 22: 737 - 744.].
- Walsh, Christopher (1989) Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. [http://www.jbc.org/content/264/5/2393.long|Journal of biological chemistry 264: 2393 - 2396.]
- Wickstrum, J.R., Santangelo, T.J., and Egan, S.M. (2005) Cyclic AMP receptor protein and RhaR synergistically activate transcription from the L-rhamnose-responsive rhaSR promoter in Escherichia coli. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251584/?report=reader|Journal of Bacteriology 187: 6708 – 6719.].
 "
"