Team:TU-Munich/Results/Summary
From 2013.igem.org
(→What to do?: Remediation) |
(→Choice of the appropriate chassis for a water filter) |
||
| Line 9: | Line 9: | ||
During this summer we wanted to work on an iGEM project which has the potential to become a real world application as we believe that this is an important step for synthetic biology to provide superior solutions for global problems. For this reason we have focused on Bioremediation: The usage of organisms to remove emissions caused by humans and to bring the environment back to its natural state. As water is a resource absolutely essential for humans, we decided to focus on the pollution of aquatic ecosystems. | During this summer we wanted to work on an iGEM project which has the potential to become a real world application as we believe that this is an important step for synthetic biology to provide superior solutions for global problems. For this reason we have focused on Bioremediation: The usage of organisms to remove emissions caused by humans and to bring the environment back to its natural state. As water is a resource absolutely essential for humans, we decided to focus on the pollution of aquatic ecosystems. | ||
==Choice of the appropriate chassis for a water filter== | ==Choice of the appropriate chassis for a water filter== | ||
| - | Remediation is not new to iGEM but instead a topic which the iGEM community worked on over nearly | + | Remediation is not new to iGEM but instead a topic which the iGEM community worked on over nearly 10 years. Therefore a set of promising BioBricks was already present in the Parts registry which we wanted to use in order to increase the knowledge on these effector proteins. Having some first effector proteins we discussed from the first minute on the best chassis for this application. Most of the previous projects on Bioremediation were based on ''E. coli'' but we decided instead to use a plant. The photosynthesis carried out by the plants will allow the water filter afterward to maintain and renew itself without the addition of nutrients. We considered alga such as Chlamydomonas reinhardtii, Bryophytes such as ''Physcomitrella patens'' and higher plants like ''Arabidopsis thaliana''. In the end ''Physcomitrella patens'' was the chassis of choice as it already grows in a filter-like structure, can be cultivated in terrestric as well as und aquatic conditions and it is a well established biotechnological organism. Working with Physcomitrella is not easy as from the transformation to the experiments with stable transfected plants 1-2 month are required and the doubling time is in the range of 3-6 days. As nobody at the TU Munich works with the moss Physcomitrella patens, we looked for an expert and found Prof. Reski from Freiburg University who helped us during our project. For the usage of Physcomitrella in iGEM we created a strong constitutive promoter ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1159306 BBa_K1159306]), a plant terminator ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1159307 BBa_K1159307]) and an antibiotic selection marker ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1159308 BBa_K1159308]) which were all used to transform and select 21 different transgenic moss lines. |
==Localization of effector proteins== | ==Localization of effector proteins== | ||
Revision as of 01:00, 29 October 2013
What to do?: Remediation
During this summer we wanted to work on an iGEM project which has the potential to become a real world application as we believe that this is an important step for synthetic biology to provide superior solutions for global problems. For this reason we have focused on Bioremediation: The usage of organisms to remove emissions caused by humans and to bring the environment back to its natural state. As water is a resource absolutely essential for humans, we decided to focus on the pollution of aquatic ecosystems.
Choice of the appropriate chassis for a water filter
Remediation is not new to iGEM but instead a topic which the iGEM community worked on over nearly 10 years. Therefore a set of promising BioBricks was already present in the Parts registry which we wanted to use in order to increase the knowledge on these effector proteins. Having some first effector proteins we discussed from the first minute on the best chassis for this application. Most of the previous projects on Bioremediation were based on E. coli but we decided instead to use a plant. The photosynthesis carried out by the plants will allow the water filter afterward to maintain and renew itself without the addition of nutrients. We considered alga such as Chlamydomonas reinhardtii, Bryophytes such as Physcomitrella patens and higher plants like Arabidopsis thaliana. In the end Physcomitrella patens was the chassis of choice as it already grows in a filter-like structure, can be cultivated in terrestric as well as und aquatic conditions and it is a well established biotechnological organism. Working with Physcomitrella is not easy as from the transformation to the experiments with stable transfected plants 1-2 month are required and the doubling time is in the range of 3-6 days. As nobody at the TU Munich works with the moss Physcomitrella patens, we looked for an expert and found Prof. Reski from Freiburg University who helped us during our project. For the usage of Physcomitrella in iGEM we created a strong constitutive promoter ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1159306 BBa_K1159306]), a plant terminator ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1159307 BBa_K1159307]) and an antibiotic selection marker ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1159308 BBa_K1159308]) which were all used to transform and select 21 different transgenic moss lines.
Localization of effector proteins
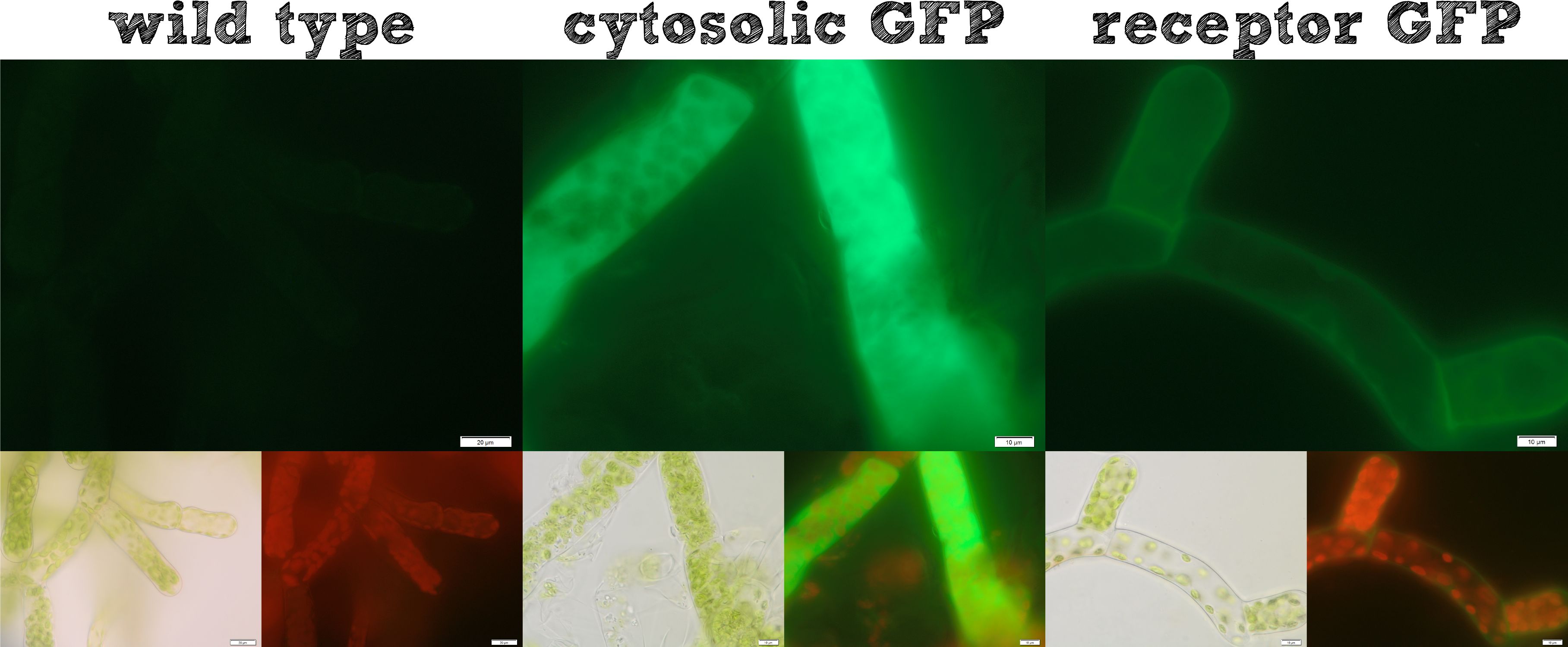
The actual remediation of pollutants is accomplished by effector proteins which are working on quite different mechanisms. Thus it was important to enable the localization of effector proteins at different cellular sites. Cytosolic effector proteins are simply expressed, whereas for secretion a signal peptide BioBrick is cloned ahead of the effector protein. Several receptor signal peptides from Physcomitrella patens were analyzed by bioinformatics tools and the signal peptide of the Somatic Embryogenesis receptor-like kinase (SERK) and the IgK signal peptide von mouse which is described in literature to function in Physcomitrella were chosen. The secretion of a novel introducted luciferase with both of these two signal peptides was investigated for 8 clones each and showed no detectable secretion for the IgKappa but a high secretion rate for the SERK signal peptide. Thus successful secretion could be achieved using the SERK signal peptide. As the secreted effector protein are not attached to the moss cell they would diffuse into the water and this might not be advantageous for example if the effector protein is a binding protein. Therefore we designed a modular receptor for Physcomitrella which can carry an effector protein at the outer side of the cell membrane. For this purpose again bioinformatics methods were used and the SERK trans membrane domain was designed. A receptor composed of (1) the SERK signal peptide, (2) an extracellularly located effector protein, (3) a linker with a Strep-tag II and a TEV protease cleavage site, (4) the SERK trans membrane domain, (5) a short linker domain and (6) a green fluorescent protein were assembled using RFC[25] standard. This highly modular receptor was successfully transformed into Physcomitrella and stable cell lines were used for experiments. The localization of the cytosolic GFP was detected at the membrane of the moss cells compared to the cytosolically expressing moss cells which showed an uniform fluorescent over the whole cell showing that our modularized receptor works. Further we incubated the moss cells with recombinant TEV protease which diffused through the cell wall, cleaves the TEV site within the extracellular domain of the receptor and liberated the NanoLuc luciferase. The luciferase assay of the supernatant at the beginning of this incubation and after one hour showed a drastic increase in luminescence which is another important argument that our modular receptor is located in the membrane and even better in the right orientation. Beyond the possibility to locate an effector protein in the extracellular part of the receptor we thought about further applications and found the SypCatcher-SpyTag System to be a perfect tool for our needs http://www.ncbi.nlm.nih.gov/pubmed/22366317 Zakeri et al., 2012. In this system a peptide bond is formed between the side chains of two protein domains in an efficient manner. With this system it is no more necessary to fuse effector proteins to a specific terminus of the receptor, it becomes possible to immobilize effector proteins which are active as multimeric proteins and it would also be possible to express a single receptor carrying a SpyCatcher domain at the outer side of the membrane which subsequently binds a set of different effector proteins which are secreted and become immobilized afterwards. Constructs were created for a His-tagged SypCatcher, a SypTag with a N-terminal or C-terminal SpyTag or with protein domains on both termini of the SpyTag. These constructs were produced recombinantly in E. coli and the proteins were then purified. Protein coupling experiments were performed and afterwards the formation of isopeptide bond was confirmed by pull-down experiments and in reducing SDS-PAGE. This system enables you as a potential user of our modular receptor to customize our receptor to any need an effector protein could have. Taken together all our intended localization BioBricks worked in Physcomitrella empowering the iGEM community to work creatively with Phsycomitrella as a chassis.
BioDegradation

Under the headline BioDegradation we investigated effector proteins which degrade pollutants by enzymatic catalysis. For this purpose we introduced the new enzyme BioBrick Erythromycin Esterase (EreB) which degrades macrolide antibiotics. On the other hand we used well established BioBricks for BioDegradation. We improved the Laccase from Bacillus pumilus by converting it to RFC[25] in order to be able to integrate it into the extracellular portion of our receptor. Both enzymes were produced as recombinant proteins and were purified. Enzymatic characterization was carried out concerning substrate dependency, salt tolerance and pH dependency. For the laccase additionally the temperature dependency and the half-life was estimated in river water. All data was fitted in our enzyme kinetic modeling. The aim was to analyze these data and to provide a solid base for our filter calculator. This calculator uses all data produced in our enzyme characterization to extrapolate for the use of transgenic PhyscoFilter in waste water treatment plants or rivers. We assumed the secretory production of laccase by our moss as the laccase degrades a wide variety of important pollutants such as the pain killer diclofenac, the oral contraceptive ethinylestradiol or iodined x-ray contrast media which are all present in nature and are hardly degradable by conventional methods. From the modeling with this calculator we learned that factor such as the degree of pollution of a river, the average temperature in a specific country, the enzyme half-life as well as the actual amount of secreted protein play an important role for the efficacy of our PhyscoFilter. Generally the results show that approximately an area of 20 football field would be required to produce enough laccase to reduce the contamination of a river with the mentioned pharmaceutical compounds. Beside this result we also produced several different stable transgenic moss lines for our BioDegradation module and could show that our cytoplasmatically expressed Erythromycin Esterase B enables our moss to degrade the macrolide antibiotic Erythromycin which is normally only degraded poorly. This experiment was measured with mass spectrometry coupled to liquid chromatography (LC-MS) and worked for the recombinant protein as well as for the transgenic plant giving the proof of principle for Physcomirella as a bioremediation organism.
BioAccumulation
Beside the enzymatic degradation of pollutants we found different methods to bind pollutants to our moss filter which we called BioAccumulation. The most obvious idea was to use binding proteins which are developed for human therapy such as antibodies for example and we used an alternative binding scaffold engineered to bind fluorescein called FluA as it has a very high affinity, a small size and a robust fold http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005. Beside this engineered binding protein we also found the idea of Dundee iGEM 2013 quite interesting to use a the protein which is affected in the toxicity mechanism of microcystein and to use it as a binding partner which then absorbs the pollutant from the water. Thus we contacted Dundee iGEM, told them about our idea of an collaboration, got their BioBrick sent, converted it to RFC[25], assembled it into our modular receptor and finally transformed and selected stable transfected moss lines which we characterized finally. Basically the limitation of BioAccumulation applications is that they only can bind one pollutant per binding protein and thus an extremely high number of binding proteins is required to achieve a reduction of environmental pollutants. We transformed and selected transgenic moss lines with all three effector proteins and checked the cellular localization of these proteins using light microscopy. For the moss lines with a receptor harboring an Anticalin which binds fluorescein a membrane bound localization could be confirmed whereas the moss lines with a receptor carrying the protein phosphatase 1 (PP1) from our collaboration partner Dundee showed a localization in cytosolic vesicles and not on the membrane. This is in good agreement with the result presented by Dundee that the SEC-pathway secretion is not working for this BioBrick. This might be because of surface exposed cystein residues which tend to aggregation in the oxidizing milieu, therefore it would be necessary to perform protein engineering to exchange these cystein residues for other amino acid residues in order to increase the stability of this protein.
Kill-Switch
Safety is one of the most important issues in synthetic biology and we therefore implemented a kill switch into our project. For us it was important to use a trigger which is ubiquitously present in the environment and no action of humans is required. We therefore developed a light-triggered kill switch. With this system the transgenic moss could be cultivated under blue filter foil. As long as the moss grows under this blue foil no red light reaches the moss and the moss stays alive. As soon as the moss escapes from this protected environment the red light is present and the kill switch becomes triggered. The system was modularized into a sensor module and a suicide module. The sensor domain consists of a splitted TEV protease which is attached to either PhyB or PIF. The later two proteins dimerized when red light is present and therefore lead to the reconstitution of the TEV protease. The suicide module consists of nuclease which is localized at the cellular membrane by a linker which contains a nuclear localization signal (NLS) and a TEV cleavage site. As soon as the sensor module is reconstituted by red light, the TEV protease cleaves its cleavage site inside the suicide module. Thereby the nuclease is liberated, becomes transported to the nucleus because of the nuclear localization signal and fragments the genome. The choice to use a nuclease instead of siRNA for example was driven by our modeling in which we found the siRNA suicide module to be less effective as there is a negative feedback loop which avoids the efficient killing of moss cells. We have transformed moss cells with this kill switch and have protected the resulting cells by the blue foil mentioned before. When we opened the blue foil after the selection process, all moss cells were dead. This can be explained by a drastically reduced transformation efficacy as the kill switch DNA was >10 kDa or by the fact that the kill switch is reliably killing the cells even without a trigger. In order to test the sensor module in vitro we have produced the two fusion proteins in vitro as recombinant proteins and have attempted to purify them which was not successful as the proteins are most probably not stable in vitro. Although we only had a single shot to test our kill switch in Physcomitrella we have discussed by far more about this system compared to the other parts of our project that worked very well in first experiments. During these discussions on our kill switch we have learned a lot about this system and we described these findings in order to help subsequent iGEM teams which are aiming to design a comparable kill switch.
Implementation
Projects in iGEM must not stop at the lab door and therefore it is immensely important to think about technical solutions to implement the transgenic organisms in order to show highest efficacy. For this reason we convinced experts like Prof. Dr. Posten to join our Counsel:_An_overview advisery board and have evaluated different cultivation methods for moss such as closed tube reactors, open pond reactors and floating remediation rafts. We came to the conclusion that in the case of immobilized effector proteins an open pond or closed tube reactor will be the superior technology as the degradation requires a close contact between the moss and a the pollutant to degrade. As a second possibility we evaluated the secretion of effector proteins such as laccase which would then be implemented best on floating remediation rafts which are cheap to produce, mobile and could also be applied in third world countries. PhyscoFilter moss could be grown on these rafts and would secrete recombinant protein which then is liberated and can degrade pollutants in the environment. For all these cultivation methods we built model reactors and also tried the cultivation of moss and the flow characteristics. For the triangular remediation raft we constructed a life-size prototype which costed only US$ 70. Additionally we developed a measurement device based on an Arduino microcontroller which measures environmental parameters, sends the data via WiFi to a computer, which then sends it to a webserver from where the actual data can be monitored with any smart phone at any place in the world.
References:
http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 Vopel S, Mühlbach H, Skerra A. (2005) Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. Biol. Chem., 386(11):1097-104.
http://www.ncbi.nlm.nih.gov/pubmed/22366317 Zakeri et al., 2012 Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M. (2012). Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci U S A. 20;109(12)
 "
"



AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351