Team:Bielefeld-Germany/Biosafety/Biosafety System
From 2013.igem.org
| Line 106: | Line 106: | ||
===Logical gate=== | ===Logical gate=== | ||
<p align="justify"> | <p align="justify"> | ||
| - | Besides the genetical approach the constructed Biosafety-System can also function as a logical gate, as shown in Figure 4. So only when the alanine racemase (Alr) is expressed the bacteria grow (E = 1) and do not when the RNase Ba is expressed (A = 1) or the alanine racemase repressed (C = 0). This concept can be extended with the addition of substances like D-alanine or an inducer, like L-arabinose for the Biosafety-System AraCtive (Figure 5). | + | Besides the genetical approach the constructed Biosafety-System can also function as a logical gate, as shown in Figure 4. So only when the alanine racemase (Alr) is expressed the bacteria grow (E = 1) and do not when the RNase Ba is expressed (A = 1) or the alanine racemase repressed (C = 0). This concept can be extended with the addition of substances like D-alanine or an inducer, like L-arabinose for the Biosafety-System AraCtive (Figure 5). In this case, on the one hand, the bacteria grow (F = 1) when the alanine racemase BBa_K1172901 is expressed (C = 1) or D-alanine is supplemented (D = 1) to the medium, allowing bacterial growth also in the absence of L-rhamnose. On the other hand, the bacteria do not grow (F = 0), when induced by an inducer (B = 1), like L-arabinose, the expression of the RNase Ba (A = 1) BBa_K1172904, or the absence of D-alanine..</p><br> |
[[File:Team-Bielefeld-Biosafety-Systems-logical-gates.png|600px|thumb|center|'''Figure 4:''' Logical gate of the double kill-switch mechanism. Inputs are represented by the expression of the Barnase (A) and alanine racemase (C), while the output represents the bacterial growth.]] | [[File:Team-Bielefeld-Biosafety-Systems-logical-gates.png|600px|thumb|center|'''Figure 4:''' Logical gate of the double kill-switch mechanism. Inputs are represented by the expression of the Barnase (A) and alanine racemase (C), while the output represents the bacterial growth.]] | ||
Revision as of 03:39, 29 October 2013
Biosafety System
Overview
Biosafety is an important aspect of Synthetic Biology, because it deals with the interaction of the environment and mankind. Several studies deal with the interaction of genetically modified bacteria and natural wild types (Snow et. al 2005). In most cases it was found that genetically modified bacteria does not influence the environment, but there is always a risk remaining (Myhr et. al 1999). Because the genetically modified bacteria are adapted to the excellent conditions of the laboratory, the natural bacteria will usually outlast these modified strains in nature due to their better adaptation to their environment. But there is no guarantee that there is really no interaction and that their release does not affect the equilibrium of the environment. To avoid these problems, we also thought of a cell free fuel cell based on enzymes, but this is even more complex and would not be as efficient because of the limited activity and lifetime of the enzymes. So the question was: how to design our Microbial Fuel Cell containing genetically modified bacteria for a higher production of energy so it can one day be applied outside the laboratory without adversely affecting nature?
The answers of this question can be found in our Safety-System. This new approach opens the possibility of controlling the cell division of living bacteria in a defined environment like the MFC. The Biosafety-System ensures that the genetically modified bacteria can only survive in the defined habitat and will die when they leave this area by accident or damage of the MFC chassis.
Basically, our Biosafety-System takes advantage of two common biosafety ideas, namely an auxotrophic strain and a toxic gene product. Our approach combines both in only one system. The constructed Biosafety-System takes the best of this two approaches by establishing an new useful auxotrophy and a new toxic gene product for the parts registry and is additional characterized as the first registered double kill-switch system. By using two different kill-switches, each individual switch can act as a backup for the other and our Biosafety-System additionally provides additional a higher plasmid stability and a higher resistance towards undesirable mutations in the Safety-System. In short: Our Biosafety-System is safe!
Theory
Genetic approach
The auxotrophic Safety-Strain E. coli K-12 ∆alr ∆dadX is the basis of our three Safety-Systems Lac of Growth, TetOR alive and AraCtive. In this new constructed Safety-Strain the constitutive alanine racemase (alr) and the catabolic alanine racemase (dadX) are deleted. As the alanine racemases catalyze the reversible isomeration from L-alanine into the enantiomer D-alanine, the Safety-Strain is no longer able to synthesize the essential amino acid D-alanine. D-alanine is an essential molecule for the Gram-negative bacteria E. coli, because it is responsible for the cross-linkage of the peptidoglycan layer, so that a lack of D-alanine inhibits cell division and leads to cell lysis in growing bacteria. This effect is comparable to bacteriolytic characteristic known from beta-lactam-antbiotics.
By complementation of the alanine racemase (<bbpart>BBa_K1172901</bbpart>) via plasmid, the supplementation of D-alanine is not necessary for the Biosafety-Strain carrying the plasmid. And as the complementation is essential for bacterial cell division, it can be used as an antibiotic-free selection system.
When expressing toxic gene products like the Rnase Ba (<bbpart>BBa_K1172904</bbpart>) or Colicin E2 (<bbpart>BBa_K131000</bbpart>), the maintenance of the plasmid stability is a very important parameter. And in a biosafety system it is even more important than in industrial cultivation, because the loss of the plasmid containing the biosafety system causes the whole biosafety system to fail. This situation is avoided in the Biosafety-System by the complementation of the alanine racemase in the Biosafety-Strain K-12 ∆alr ∆dadX. If these mutants loose the plasmid, caused by the stress of the toxic gene product, they will not be able to grow, because the bacteria cannot synthesize D-alanine for the peptidoglycan layer of the cell wall.
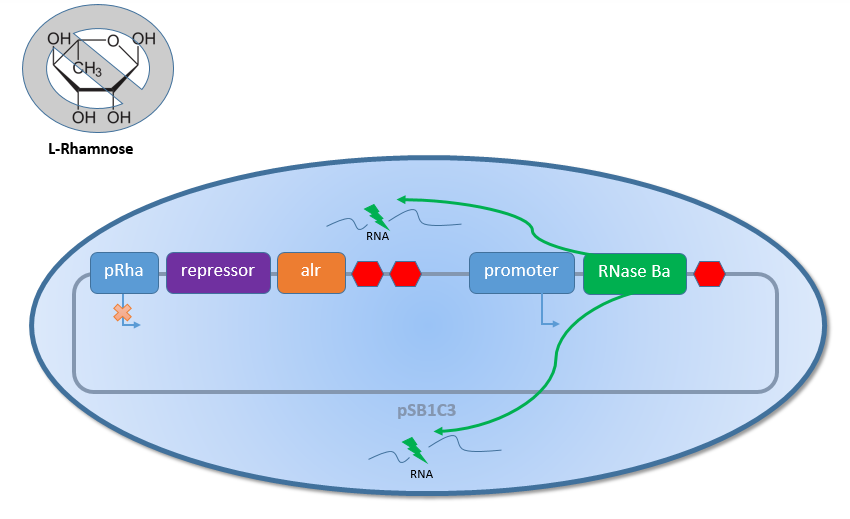
In general, the Biosafety-System can be divided into two parts, which are regulated by different inducible promoters. The first part contains the essential alanine racemase (alr) and the corresponding repressor for the promoter of the second part. Both genes a regulated by the rhamnose inducible promoter PRha (<bbpart>BBa_K914003</bbpart>). The second part of the Biosafety-System contains another promoter which depends on the Safety-System. The Biosafety-System AraCtive for example uses the arabinose promoter PBAD. This promoter controls
in the final version the expression of the toxic RNAse Ba (<bbpart>BBa_K1172904</bbpart>), but for the testing and comparison of the different Biosafety-Systems GFP (<bbpart>BBa_E0040</bbpart>) was used in all of the Systems instead. This way a selection of the best designed Biosafety-System is possible without direct natural selection by the Biosafety-System itself.
As shown in Figure 1 above, the Biosafety-Strain survives only under defined conditions by supplementation of L-rhamnose to the media. L-rhamnose induces the expression of alanine racemase, so that the bacteria can grow, and the expression of the repressor, which inhibits the expression of the toxic Barnase. To establish a functional Biosafety-System, we first compared three different System (AraCtive, TetOR alive and Lac of Growth) by variations of the repressor and the corresponding promoter to measure the basal transcription and repression of the second part. This is necessary for the first fine tuning of the Biosafety-System, as it was not clear how high the expression level of the Barnase could be without causing cell death. With GFP, it was therefore possible to get a first impression of how low the transcription of the Barnase would be in the repressed situation.
In contrast, when there is no L-rhamnose present in the media or the concentration of L-rhamnose decreases because of the natural Rhamnose catabolism of the Biosafety-Strain K-12 ∆alr ∆dadX, the expression of the first part with the alanine racemase (alr) and the repressor decreases (see Figure 2 below). This leads to an activation of the second promoter. Also for this situation the testing with GFP was necessary before the usage of the toxic Barnase in order to get an impression how high the expression of the Barnase would be.
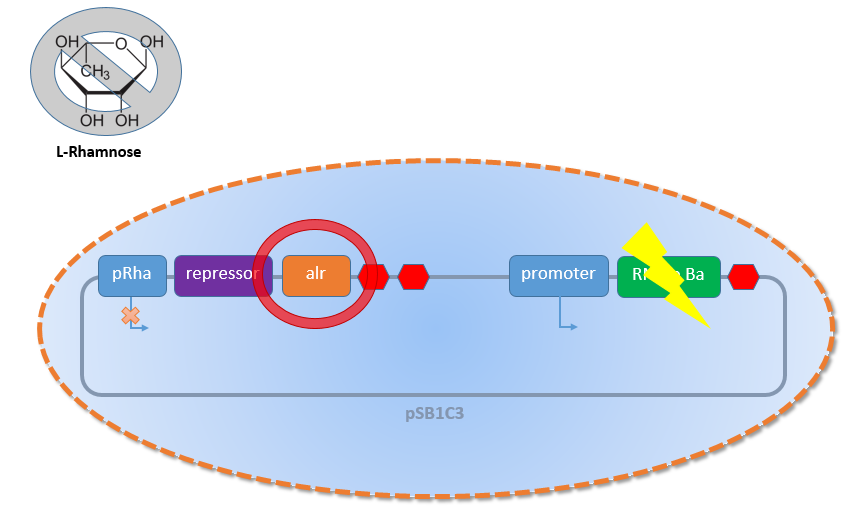
An additional benefit of the Biosafety-System is not shown in Figure 2 above. As mentioned before, a problem of biosafety systems is the possible loss of the plasmid. This problem is solved in our Biosafety-System by the complementary function of the alanine racemase. Another possible weakness of common biosafety systems is failure due to mutations in the kill gene. Even if the plasmid is present, a mutation in the toxic gene or in its promoter causing loss of gene function or expression leads to failure to kill the cells. In this situation, the existing biosafety systems are not safe anymore. Figure 4 shows the solution.
As visualized in Figure 3 above, this problem is solved in our Biosafety-System by using once again the advantage of the Biosafety-Strain E. coli K-12 ∆alr ∆dadX and the alanine racemase (alr). In addition to the induction of the toxic Barnase expression in absence of L-rhamnose, the expression of alanine racemase is shut down causing a lack of cell growth additionally to the Barnase!
This huge advantage of this double kill-switch makes the whole Safety-System much more resistant against mutations and therefore safe. The mutations that are not affecting the safety of the system underlie the natural selection and disappear. For example a mutation in the repressor, which leads to its inactivation, will causes cell death by activation of the toxic Barnase, or a mutation of the alanine racemase will stop the cell division. But any mutation that normally affect the safety of the system and will lead to failure and can be abolished by the double kill-switch. This concerns mutations that will shutdown the toxic effect of the Barnase by mutation in the promoter of the second part or the Barnase itself. An inactivation of the Barnase can be compensated by the repressed alanine racemase (Alr) in the absence of L-rhamnose. So the only weak point in the Biosafety-System occurs when the rhamnose promoter PRha becomes constitutive. As this sequence is only 200 bp short and the chance that the rhamnose promoter PRha will become constitutive is very, very low, because it is tightly shutdown naturally and only activated positively by L-rhamnose. So in conclusion the Biosafety-System is safe!
Logical gate
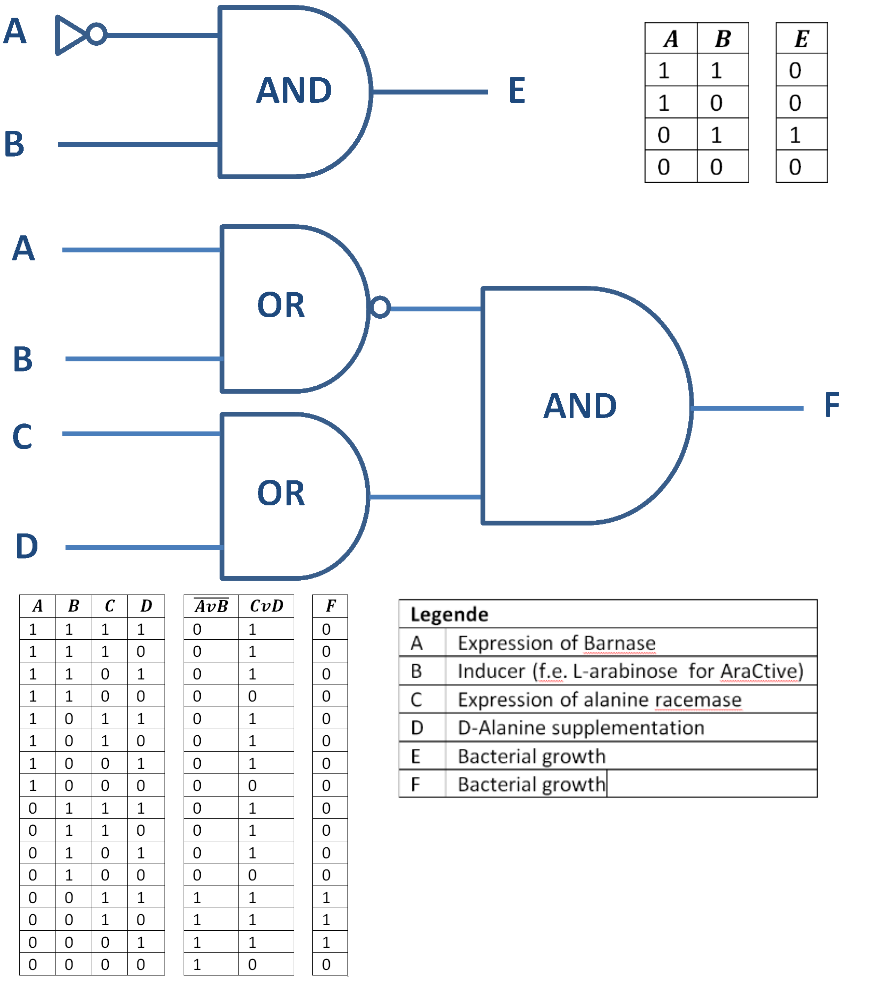
Besides the genetical approach the constructed Biosafety-System can also function as a logical gate, as shown in Figure 4. So only when the alanine racemase (Alr) is expressed the bacteria grow (E = 1) and do not when the RNase Ba is expressed (A = 1) or the alanine racemase repressed (C = 0). This concept can be extended with the addition of substances like D-alanine or an inducer, like L-arabinose for the Biosafety-System AraCtive (Figure 5). In this case, on the one hand, the bacteria grow (F = 1) when the alanine racemase BBa_K1172901 is expressed (C = 1) or D-alanine is supplemented (D = 1) to the medium, allowing bacterial growth also in the absence of L-rhamnose. On the other hand, the bacteria do not grow (F = 0), when induced by an inducer (B = 1), like L-arabinose, the expression of the RNase Ba (A = 1) BBa_K1172904, or the absence of D-alanine..
Results
Following the results of the three Biosafety-Systems AraCtive, TetOR alive and Lac of Growth a compared together to get an impression of their difference. Therefore, the metabolic pressure of the Biosafety-Plasmids, their basal transcription and the kill-switch mechanism were simulated with GFP <bbpart>BBa_E0040</bbpart>.
Figure 5 compares the cultivation process of the Biosafety-Strain E. coli K-12 ∆alr ∆dadX containing the corresponding Biosafety-Plasmid. The cells were cultivated in M9 minimal medium with glycerol and 1% L-rhamnose to measure the metabolic pressure caused by the Biosafety-Plasmid. In the presence of L-rhamnose, the expression of the repressor and the alanine racemase (Alr) is induced, while the expression of the toxic gene product is repressed. Compared to the uninduced Biosafety-Systems the expression of these genes causes a higher energy expenditure for the cell, but as demonstrated in Figure 6 there is no significant difference between the Biosafety-Systems. So metabolic pressure is not a criteria for the preference of one of the Biosafety-Systems.
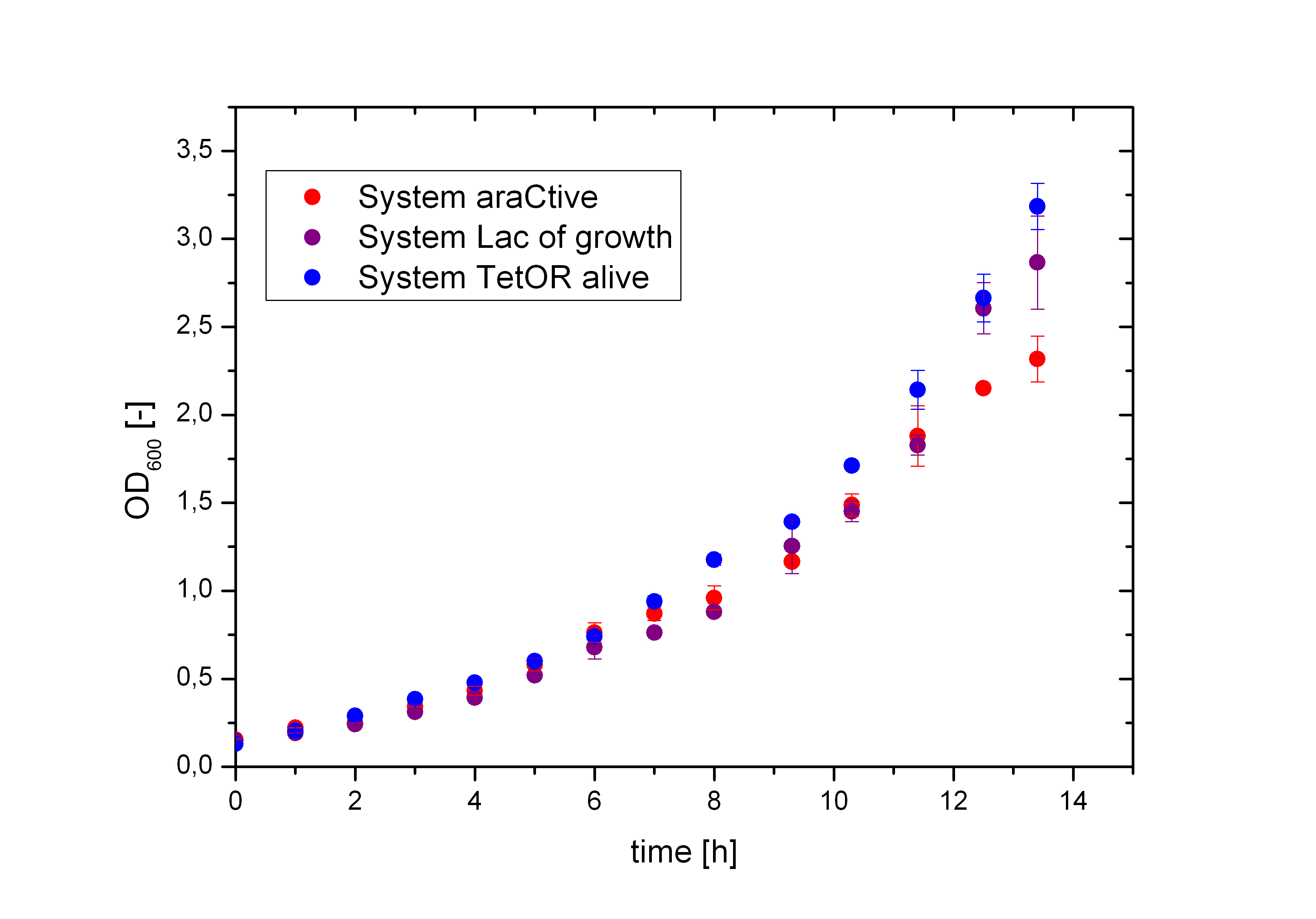
In Figure 6, the basal transcription of the three Biosafety-Systems is analyzed by comparison of the specific production rate of GFP. This is important to get an impression of the lethality of each Biosafety-System, because a higher basal transcription will result in a higher expression of the toxic RNase Ba <bbpart>BBa_K1172904</bbpart>. Therefore, this parameter is even more important for the function of the Biosafety-System. Figure 6 demonstrated that the Biosafety-System AraCtive is characterized by the lowest basal transcription, followed by the Biosafety-System Lac of Growth and TetOR alive. This means that the Biosafety-System AraCtive <bbpart>BBa_K1172909</bbpart> would be the most potent one, as the toxic gene product will have the lowest effect and therefore the growth rate will be the highest in this System.
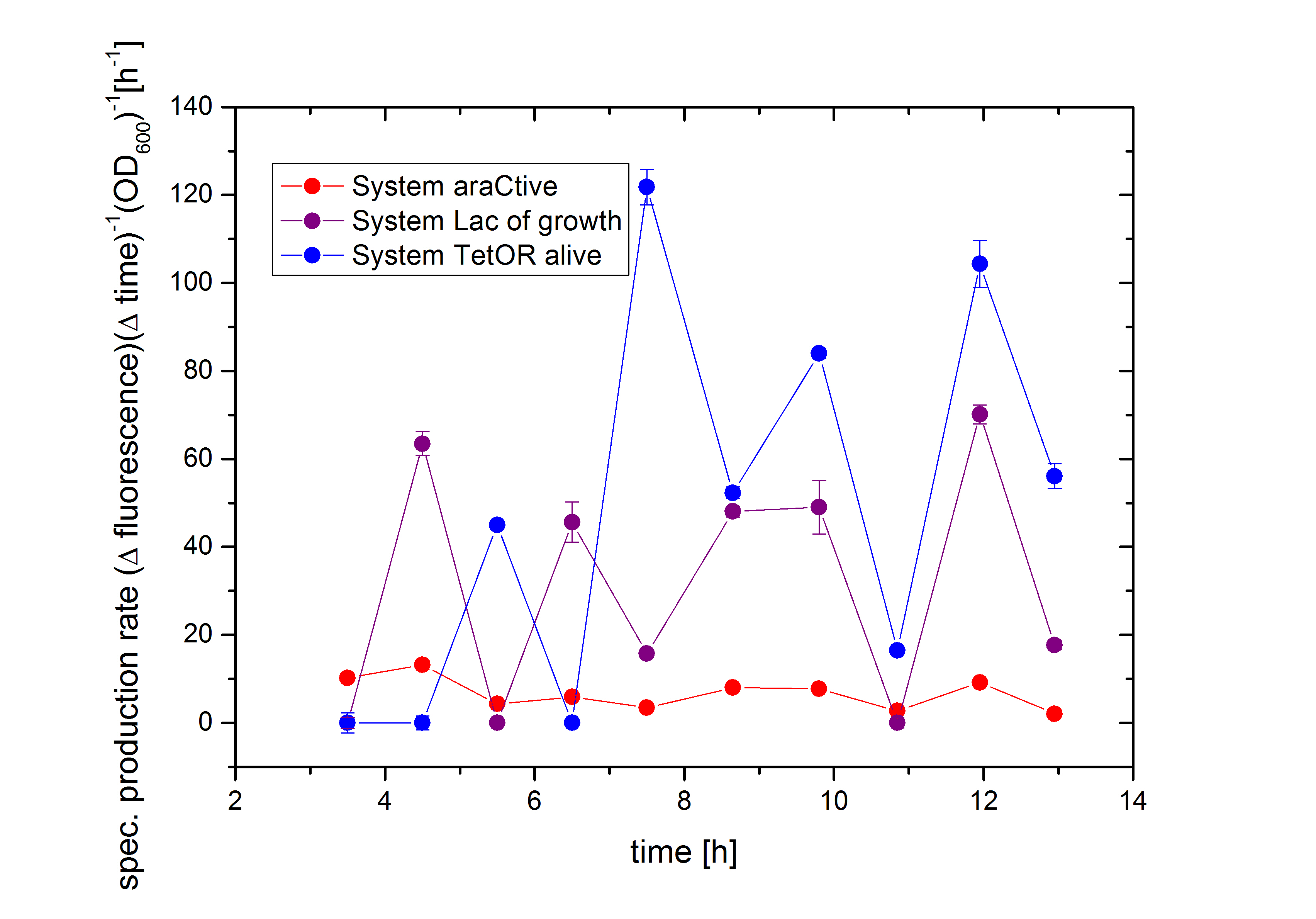
Moreover, the kill-switch mechanism is another important characteristic of the Biosafety-Systems. This parameter can be measured by difference in the expression levels of GFP. An ideal Biosafety-System would show a minimal basal transcription in the presence of L-rhamnose and a high expression level in its absence to ensure the kill mechanism. The kill-switch mechanisms for the three Biosafety-Systems are shown in Figure 7. All Biosafety-Systems were cultivated in M9 minimal medium. The bars marked with a star are additional induced with 1% L-rhamnose, resulting in the expression of the first part, the repressor and the alanine racemase. It can be seen that the Biosafety-System AraCtive with the lowest basal transcription (orange bar) shows a higher expression in the absence of L-rhamnose (red bar), but the activation is not as strong compared to Biosafety-System Lac of Growth. This Biosafety-System is characterized by a higher basal transcription in the presence of L-rhamnose (light blue bar), but a potent activation in its absence (dark blue bar). The Biosafety-System TetOR alive shows the highest basal transcription and the activation differs not that much during cultivation. Because the TetOR-System is actually known for an effective switch mechanism, the optimizations of the Biosafety-System are discussed and needs optimizations before an adequate analysis is possible.
The induction of the Biosafety-Systems AraCtive and Lac of Growth with L-rhamnose leads to a repression of the expression of GFP when compared to the uninduced state. Therefore the second part of these Biosafety-Systems, which contains normally the toxic RNase Ba <bbpart>BBa_K1172904</bbpart> can be used as a kill-switch regulated by the pentose L-rhamnose. So it could be demonstrated that these Biosafety-Systems work in principle. Because of the tight repression of the second part by the arabinose promoter PBAD the Biosafety-System AraCtive would be probably the best choice at the moment. But there exists also powerful ideas for the others Biosafety-Systems like creating a double lac promoter to tighten the basal transcription and generating an Biosafety-System with a tight repression and a strong activation.
Another possibility could be the utilization of the Inhibitor Barstar instead of any repressor (Mossakowska et. al, 1989). In this case are very tight repression of the Barstar is needed, when the cell leave the defined environment, to activate the Barnase. These approach probably open the possibility for a tight inhibition of the toxic gene product RNase Ba, but the activation of the Barnase might be very slow, because on the one hand the rhamnose promoter PRha is continuously shut down, so that the inhibitor amount decreases only slowly and one the other hand the inhibitor Barstar is characterized by a slow degradation. A possible solution might be the introduction of a SsrA [http://parts.igem.org/Protein_domains/Degradation degradation tag] at the C-terminus of Alr (Levchenko et. al, 2003).
This approach might also useful for the degradation of the alanine racemase, because it can be seen, that all Biosafety-Systems can also grow without L-rhamnose induction, so that the double-kill switch mechanism is not ideal at this moment and a tighter repression of the alanine racemase is necessary to profit from the double-kill switch mechanism. As the high copy plasmid pSB1C3 was used for all Biosafety-Systems, it is discussed that an adjustment of the copy number might improve the double-kill switch mechanism. Moreover the lower plasmid copy number might simplify also the integration of the Barnase instead of GFP in the Biosafety-System.
Although the double-kill switch mechanism is not that effective, the constructed Biosafety-Systems AraCtive and Lac of Growth we constructed can already be used as a kill-switch Biosafety-System and because of the essential alanine racemase they are characterized by a higher plasmid stability compared to common Biosafety-Systems. The suggestions above could result in the first double-kill switch Biosafety-System which in combination with an effective toxic gene product like the Barnase <bbpart>K1172904</bbpart> result in a powerful Biosafety-System with a reduced remaining risk compared to common Biosafety-Systems.
References
- Snow A., Andow D., Gepts P., Hallerman E., Power A., Tiedje J. and Wolfenbarger L. (2005) Genetically Engineered Organisms And The Environment: Current Status And Recommendations. [http://dx.doi.org/10.1890/04-0539 Ecological Applications 15:377 – 404].
- Levchenko I., Grant R., Wah D., Sauer R., Baker T. (2003) Structure of a delivery protein for an AAA+ protease in complex with a peptide degradation tag. [http://ac.els-cdn.com/S109727650300323X/1-s2.0-S109727650300323X-main.pdf?_tid=4fe9541a-3fed-11e3-a15a-00000aab0f01&acdnat=1382977603_a779ff84590dc557902de5503c7e433a|Molecular cell 12:365 - 72.]
- Mossakowska, Danuta E. Nyberg, Kerstin and Fersht, Alan R. (1989) Kinetic Characterization of the Recombinant Ribonuclease from Bacillus amyloliquefaciens (Barnase) and Investigation of Key Residues in Catalysis by Site-Directed Mutagenesis [http://pubs.acs.org/doi/pdf/10.1021/bi00435a033|Biochemistry 28: 3843 - 3850.].
- Myhr, Anne and Traavik, Terje (1999) The Precautionary Principle Applied to Deliberate Release of Genetically Modified Organisms (GMOs) [http://www.microbecolhealthdis.net/index.php/mehd/article/viewFile/7886/9230 Microbial Ecology in Health and Disease 11: 65 – 74].
 "
"