Team:Evry/Protocols/03
From 2013.igem.org
(Difference between revisions)
| Line 44: | Line 44: | ||
<h2> Test </h2> | <h2> Test </h2> | ||
| - | Before the storage of the tubes at -20°C, measure the concentration with nanodrop.<br> | + | <p>Before the storage of the tubes at -20°C, measure the concentration with nanodrop.<br><br></p> |
| - | + | ||
<div align="center"><img src='https://static.igem.org/mediawiki/2013/f/f0/Nanodrop.png' width="500px"/></div> | <div align="center"><img src='https://static.igem.org/mediawiki/2013/f/f0/Nanodrop.png' width="500px"/></div> | ||
| - | <br> | + | <br><p> |
260/280 ratio indicate ...<br> | 260/280 ratio indicate ...<br> | ||
260/230 ratio indicate ...<br> | 260/230 ratio indicate ...<br> | ||
If the concentration is between 30 and 50 ng/μL,<br> | If the concentration is between 30 and 50 ng/μL,<br> | ||
| - | If the concentration is below 30 ng/μL or if 260/280 ratio or 260/230 ratio is respectively..., make another purification.<br> | + | If the concentration is below 30 ng/μL or if 260/280 ratio or 260/230 ratio is respectively..., make another purification.<br></p> |
Revision as of 11:23, 26 August 2013
Plasmid purification
Principle
Preparation
Protocole from Macherey-Nagel plasmid purification notebook1. Cell culture
Cultivate cells in LB medium overnight.
2. Cell harvesting
Set saturated E.coli LM culture into 2 mL tubes. Centrifuge at 11 000 x g for 30 secondes. Discard as much as supernatant as possible.
3. Cell lysis
Add 250 μL Buffer A1 (resuspension buffer). Resuspend the cells with a vortex or a pipette.
Add 250 μL Buffer A2 (lysis buffer). Mix gently by inverting the tube 6 - 8 times. Incubate at room temperature until lysate appears clear.
Add 300 μL Buffer A3 (neutralisation buffer). Mix thoroughly by inverting the tube 6 - 8 times .
4. Lysate clarification
Centrifuge at 11 000 x g for 5 minutes . Repeat this step until supernatant is not clear.
5. DNA Binding
Place a NucleoSpin Plasmid Column in a Collection Tube of 2 mL et and set the supernatant from the last step. Centrifuge at 11 000 x g for 1 minute. Discard flow-through and place the column back into the collection tube.
6. Membrane washing
Add 600 μL Buffer A4 (wash buffer) previously supplemented with ethanol. Centrifuge ar 11 000 x g for 1 minute. Discard flow-through and place the column back into an empty collection tube.
7. Dry membrane
Centrifuge at 11 000 x g for 2 minutes and discard the collection tube.
8. DNA Elution
Place the column in a 1,5 mL and add 50 μL de Buffer AE (elution buffer). Incubate at room temperature and centrifuge at 11 000 x g for 1 minute.
Test
Before the storage of the tubes at -20°C, measure the concentration with nanodrop.
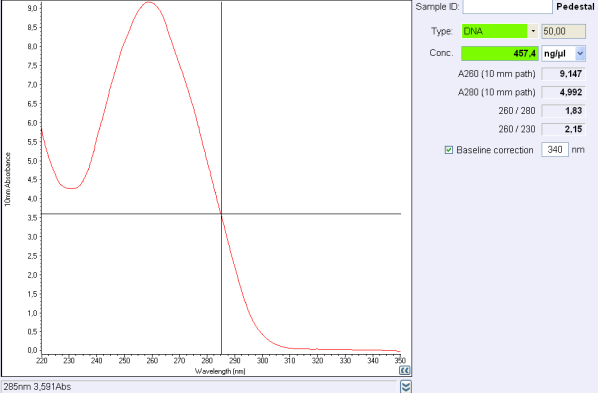
260/280 ratio indicate ...
260/230 ratio indicate ...
If the concentration is between 30 and 50 ng/μL,
If the concentration is below 30 ng/μL or if 260/280 ratio or 260/230 ratio is respectively..., make another purification.
 "
"













