Team:Bielefeld-Germany/Labjournal/July
From 2013.igem.org
July
Milestones
- Poster presentation at the congress ‘[http://www.biotechnologie2020plus.de Next generation of biotechnological processes 2020+]’ in Berlin.
9.Week
Organization
- We’ve presented us at the congress ‘[http://www.biotechnologie2020plus.de Next generation of biotechnological processes 2020+]’ in Berlin at 27. June 2013. There we have met an expert (Dr. Falk Harnisch) on the topic of MFC and we have decided to participate the 'Day of Synthetic Biology in Germany'.
MFC
Mediators
Cytochromes
- Design and order of new Primers
- The mtrCAB fragment has two illegal PstI restriction sites at 1793bp and 2078bp, so specific primers to remove them had to be designed. One base in each restriction site was replaced by respective primer overlaps without affecting the coding triplett. The three different fragments will be ligated back together and into the pSB1C3 shipping plasmid via Gibson Assembly.
- mtrCAB_Frag1_rev (30 nt): GACCTGTTTTATCTGTTGCAGCGGCAGTAT
- mtrCAB_Frag2_fwd (30 nt): ATACTGCCGCTGCAACAGATAAAACAGGTC
- mtrCAB_Frag2_rev (30 nt): GGTGTGACGACTAATGCCATAATTGCAGAC
- mtrCAB_Frag3_fwd (30 nt): GTCTGCAATTATGGCATTAGTCGTCACACC
Biosafety
10.Week
Organization
- We can present new sponsors of iGEM-Team Bielefeld: Stockmeier, Baxter, Promega, BIO.NRW and Applichem will support our team.
MFC
Mediators
- Glycerol dehydrogenase
- Further work on GldA Biobrick is needed: Plasmid restriction analysis and colony PCR could not give sufficient results.
- Successful PCR (standard Phusion PCR) on the <bbpart>BBa_J04450</bbpart> Biobrick plasmid using Forward Primer pSB1C3 and Reverse Primer pSB1C3 for later Gibson Assembly.
- Gibson Assembly with GldA PCR product and pSB1C3 PCR product.
- Screening of colonies from Gibson Assembly with colony PCR and Plasmid restriction analysis shows religated pSB1C3, as already seen while using NEB Assembly Kit.
Cytochromes
Biosafety
Porines
- We will try to use Gibson Assembly for cloning OprF into pSB1C3.
- Gibson Assembly with OprF PCR product and pSB1C3 PCR product. Screening of colonies from Gibson Assembly]] with colony PCR and Plasmid restriction analysis shows religated pSB1C3, as already seen while using NEB Assembly Kit.
11.Week
Organization
- We’ve presented us at the congress “[http://www.bio.nrw.de/studentconvention BioNRW pHD Student Convention]” in Düsseldorf at 13. July 2013 with a short presentation about iGEM and our project.
- We proudly present our [http://ekvv.uni-bielefeld.de/blog/uniaktuell/entry/mit_bakterien_batterie_strom_erzeugen first press release.] It was amazing to see how often our press release was picked up.
MFC
Mediators
- Glycerol dehydrogenase
- Repeating Gibson Assembly shows every time a religated pSB1C3, a Gibson overlap of Prefix and Suffix with 20 bp seems to be to short, furthermore Prefix and Suffix have too many homologous regions.
Cytochromes
Biosafety
Porines
- Repeating Gibson Assembly shows every time a religated pSB1C3, a Gibson overlap of Prefix and Suffix with 20 bp seems to be to short, furthermore Prefix and Suffix have too many homologous regions.
12.Week
Organization
- Dr. Falk Harnisch will have a presentation at CeBiTec colloquium as an expert for our team.
- Preparing experiments for the ‘Day of Synthetic Biology’. Our ideas are to have different experiments like DNA isolation from fruit and vegetables, pipetting of bright colors, chromatography with markers, a potato battery or microscopy.
- The TV station [http://www1.wdr.de/mediathek/video/sendungen/lokalzeit/lokalzeit-owl WDR] would like to contribute with us on our topic.
MFC
Mediators
- Glycerol dehydrogenase
- New Primerdesign for pSB1C3 with longer overlaps individually for each part in order to stop the problem of religated pSB1C3:
- Forward Primer pSB1C3 GldA (43 bp): TCCTGCAAGAGTGGGAATAATACTAGTAGCGGCCGCTGCAGTC
- Reverse Primer pSB1C3 GldA (43 bp): GATTGAATAATGCGGTCCATCTAGAAGCGGCCGCGAATTCCAG
Cytochromes
- Amplification of Fragment 1
- Size: 1800 bp
- Program: PhusionPCR
- Gradient: 54°C - 71°C over 8 steps
- Primer: mtrC_fwd & mtr_Frag1_rev
- Template: S. oneidensis PCR Template from genomic DNA
- Notes: Annealing temperature has no significant impact on the PCR
- Amplification of Fragment 2
- Size: 330 bp
- Program: PhusionPCR
- Primer: mtr_Frag2_fwd & mtr_Frag2_rev
- Template: S. oneidensis PCR Template from genomic DNA
- Notes: Annealing temperature has no significant impact on the PCR
- Amplification of Fragment 3
- Size: 3000 bp
- Program: PhusionPCR
- Gradient: 54°C - 71°C over 8 steps
- Primer: mtr_Frag3_fwd & mtrB_rev
- Template: S. oneidensis PCR Template from genomic DNA
- Notes:
- Amplification of ccmAH cluster
- Size:6311 bp
- Program: PhusionPCR
- Gradient: 51.5°C - 68°C over 8 steps
- Primer: ccmAH_fwd & ccmAH_rev
- Template: E. coli PCR Template from genomic DNA
- Notes: A high annealing temperature of 68°C or more increases yield as well as reduces unspecific bands and is therefore recommended.
- Gelextraction and cleanup of Fragment 1, 2, 3 and ccmAH
- Gelextraction and clean up with the AnalytikJena GelExtraction-Kit
- Elution buffer was preheated to 50°C
- We eluted each column twice, with 20 ul each and combined it afterwards
- Measurement of nucleid acid concentration via NanoDrop
- Fragment1: 4-2607-304: 10.5 ng/ul
- Fragment2: 4-2707-003: 44.6 ng/ul
- Fragment2: 4-2707-004: 13.5 ng/ul
- Fragment3: 4-2607-301: 11.1 ng/ul
- Fragment3: 4-2607-302: 8.9 ng/ul
- Fragment3: 4-2607-303: 9.2 ng/ul
- ccmAH: 4-2807-302: 11.0 ng/ul
- ccmAH: 4-2807-303: 11.5 ng/ul
Cytochromes
Biosafety
Porines
- New Primerdesign for pSB1C3 with longer overlaps individually for OprF part in order to stop the problem of religated pSB1C3:
- Forward Primer pSB1C3 OprF (43 bp): TTGAAGCCCAAGCTAAGTAATACTAGTAGCGGCCGCTGCAGTC
- Reverse Primer pSB1C3 OprF (43 bp): AAGGTGTTTTTCAGTTTCATCTAGAAGCGGCCGCGAATTCCAG
13.Week
Organization
- Spontaneous visit of [http://www.radiobielefeld.de Radio Bielefeld] in our laboratory. In addition to a radio interview a [http://www.radiobielefeld.de/programm/bei-uns-im-programm/studenten-entwickeln-biobatterie.html short video clip] was filmed.
MFC
Mediators
Cytochromes
Biosafety
- Amplification of different fragments:
- Recipe:
- PhusionBuffer: 5,5µL
- dNTPs: 1µL
- Primer1: 1µL
- Primer2: 1µL
- Template: 1,5 µL
- DMSO: 0,5 µL
- Phusion: 0,5 µL
- Dest. H2O: 39 µL
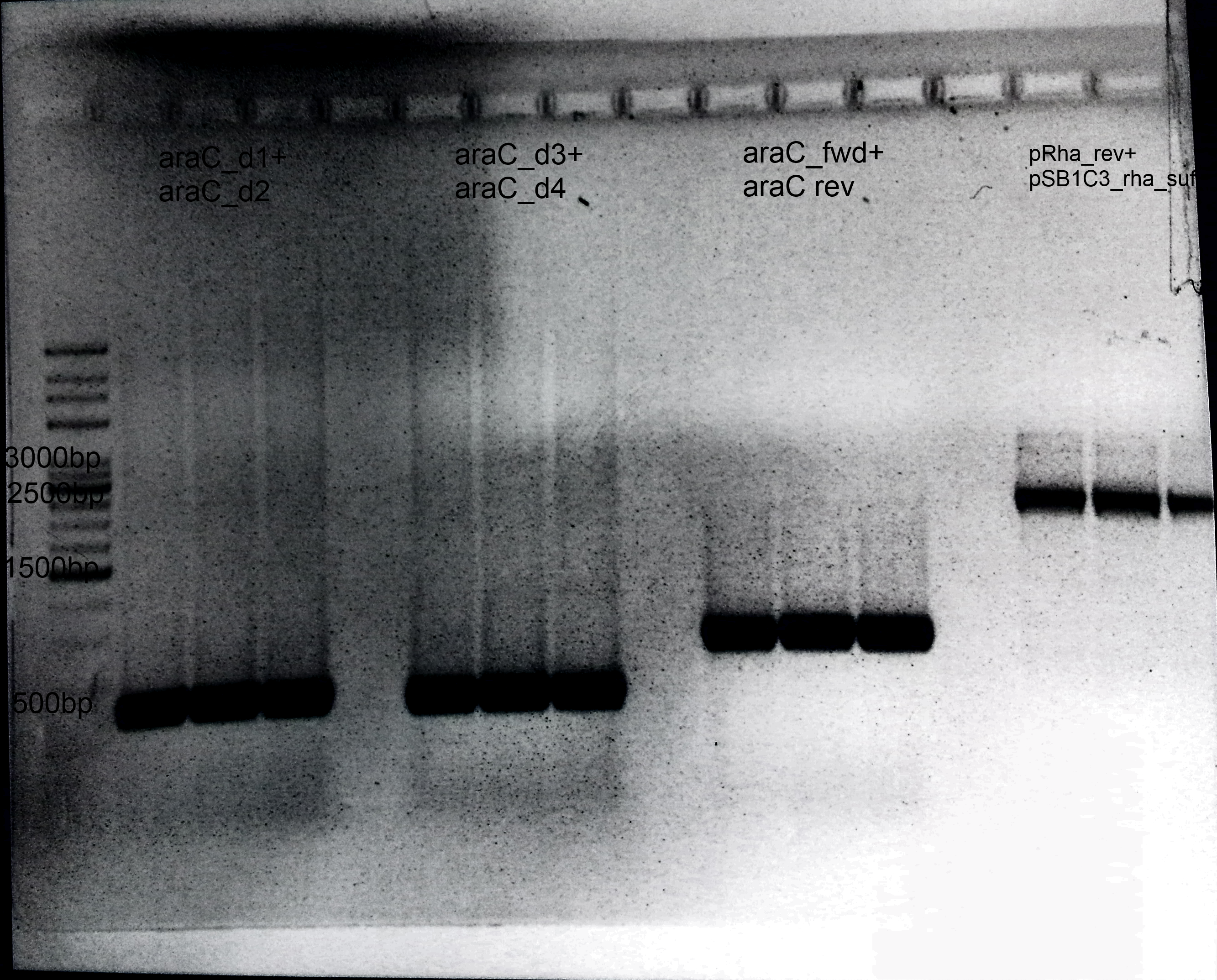
- 1.AraC:
- Primer: araC_d1+ araC_d2
- Annealing: 62°C
- Extension: 1min
- Template: E.coli Genome
- Size: 600 bp
- 2.Deletion of araC:
- Primer: araC_d3+ araC_d4
- Annealing= 62°C
- Extension: 1min
- Template: E.coli Genome
- Size: 600 bp
- 3.Amplification of araC into our Biosafety system:
- Primer: araC_fwd+ araC rev
- Annealing= 62°C
- Extension: 1min
- Template: BBa_I13458
- Size: 900 bp
- 4.Preparation of pSB1C3:
- Primer: pRha_rev+ pSB1C3_rha_suf
- Annealing: 62°C
- Extension: 1.30 min
- Template: BBa_I13541
- Size: 3 kb
- 5.Preparation of pSB1C3:
- Primer: pSB1C3_alr_fwd+ pSB1C3_alr_rev
- Annealing: 62°C
- Extension: 1 min
- Template: E.coli Genome
- Size: 1kb
- 6.Preparation of pSB1C3:
- Primer: pSB1C3_plac_alr+ pSB1C3_alr_rev
- Annealing: 62°C
- Extension: 1min
- Template: E.coli Genome
- Size: 1kb
- 7.Preparation of pSB1C3:
- Primer: pSB1C3_alr_pre+ pSB1C3_alr_suf
- Annealing: 62°C
- Extension: 1.30 min
- Template: pSB1C3
- Size: 2kb
- 8.Preparation of pSB1C3:
- Primer: pSB1C3_plac_pre+ pSB1C3_alr_suf
- Annealing: 62°C
- Extension: 1.30min
- Template: pSB1C3
- Size: 2kb
- Analysis by agarosegel
- Result:failed
- New PCR:
- Program: 06 phy_cyt mit 62°C Annealing
- 10µL 5xHF-Buffer
- 4µL dNTPs
- 0,5µL Primer1
- 0,5µL Primer2
- 1µL Template
- 1,5µL DMSO
- 1µL Enzyme
- 31,5µL dest. H2O
- Analysis by agarosegel:
- PCR-Purification:
- BS1a (9-38-451) 113,6ng/µL
- BS1b (9-38-452) 75,1 ng/µL
- BS2a (9-38-453) 85,5 ng/µL
- BS3a (9-38-454) 107,6 ng/µL
- BS4a (9-38-455) 14,4 ng/µL
- BS5a (9-38-456) 44,5 ng/µL
- BS5b (9-38-457) 49,0 ng/µL
- BS6a (9-38-458) 63,6 ng/µL
- BS7a (9-38-459) 6,9 ng/µL
- BS8a (9-38-460) 8,6 ng/µL
- BS1a (9-38-451) 113,6ng/µL
 "
"