Team:Bielefeld-Germany/Labjournal/October
From 2013.igem.org
October
Milestones
- Successful cultivations of Escherichia coli KRX with oprF and gldA in our Microbial Fuel Cell.
- NADH overproduction for the gldA strain improves extracellular electron transfer mediated by NADH and resulting in an 20 % increased bioelectricity output.
- Escherichia coli KRX with oprF shows 100 % higher voltage and electric charge than E. coli wild type. Electron shuttle-mediated electron transfer across the membrane is greatly improved by heterologous expression of outer membrane porin oprF.
- Our preferred riboflavin production strain, E. coli KRX equipped with <bbpart>BBa_K1172306</bbpart> , was thouroughly characterized.
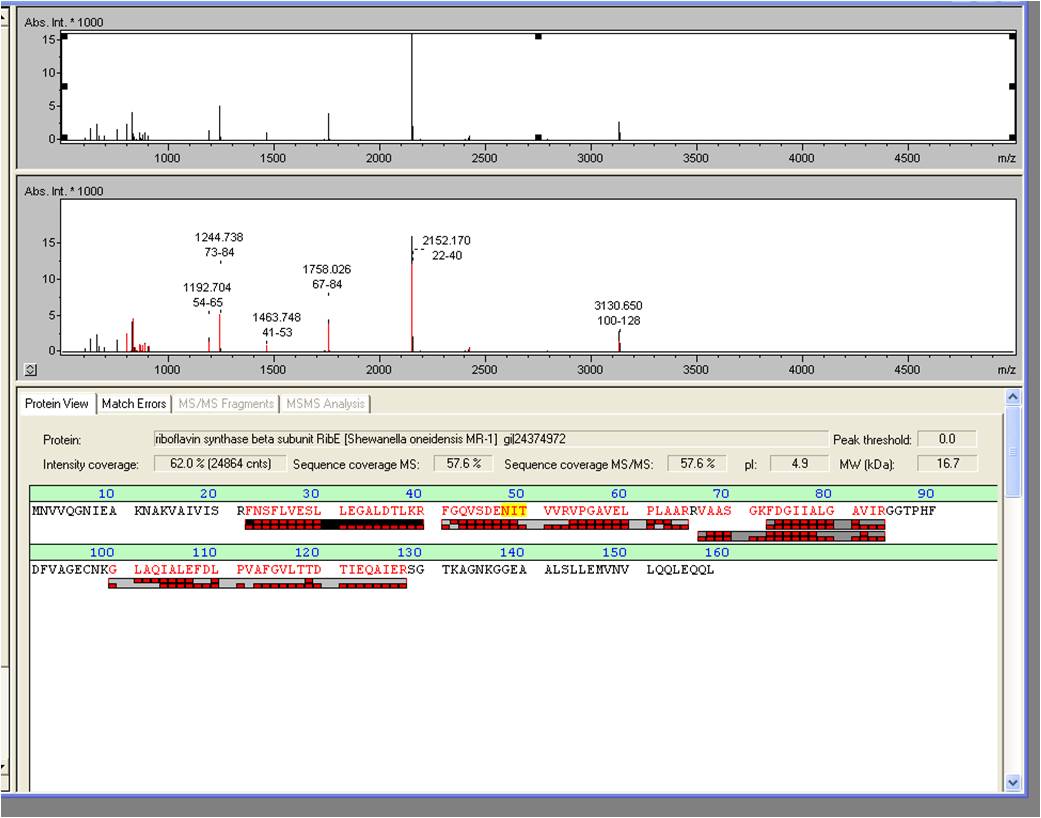
- The overexpression of Riboflavin synthase beta subunit RibE, which belongs to the rib-gene-cluster <bbpart>BBa_K1172303</bbpart> . was examined by MALDI-TOF MS/MS with a Mascot Score of 906 against the NCBI database.
- We proofed the production of riboflavin qualitatively through LC/MS measurement.
- Measuring the fluorescence of supernatant samples from our riboflavin production strain together with riboflavin solutions of known concentration enabled us to make predications regarding the amount of riboflavin produced by our strain.
- Measurement of supernatant samples from our riboflavin production strain against wild type samples with HPLC confirmed an approximately 60fold overexpression of riboflavin and backed up our result from fluorescence and absorbance measurements.
Week 22
Organization
- Wiki freeze is coming on saturday at 5:59 pm. Plannung the last week with tremendous amount of work.
MFC
Mediators
- For testing the genetic engineered system in the Microbial Fuel Cell, we used Escherichia coli KRX with gldA and Lac promotor (<bbpart>BBa_K1172204</bbpart>) in contrast to Escherichia coli KRX wild type. Certainly NADH-assays determined Escherichia coli KRX with gldA and T7 promotor (<bbpart>BBa_K1172203</bbpart>) as the best endogenous mediator producing strain. Unfortunately we could not use this strain due to cultivation problems.
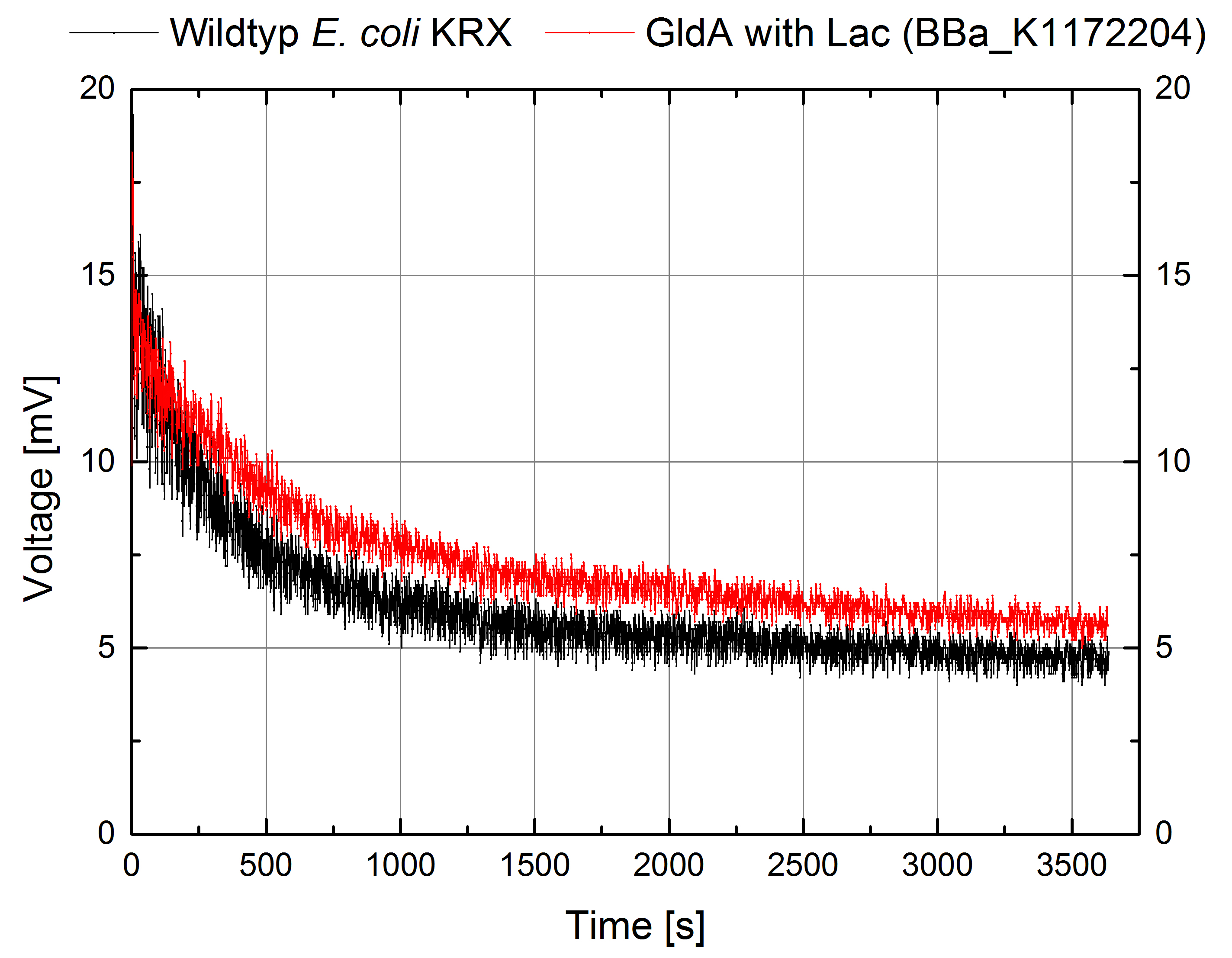
Figure 6: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with gldA (<bbpart>BBa_K1172204</bbpart>) in contrast to Escherichia coli KRX wild type. Voltage curve from Escherichia coli KRX wild type and Escherichia coli KRX with gldA (<bbpart>BBa_K1172204</bbpart>) is shown over time. M9-medium was used with no supplementation of mediators.
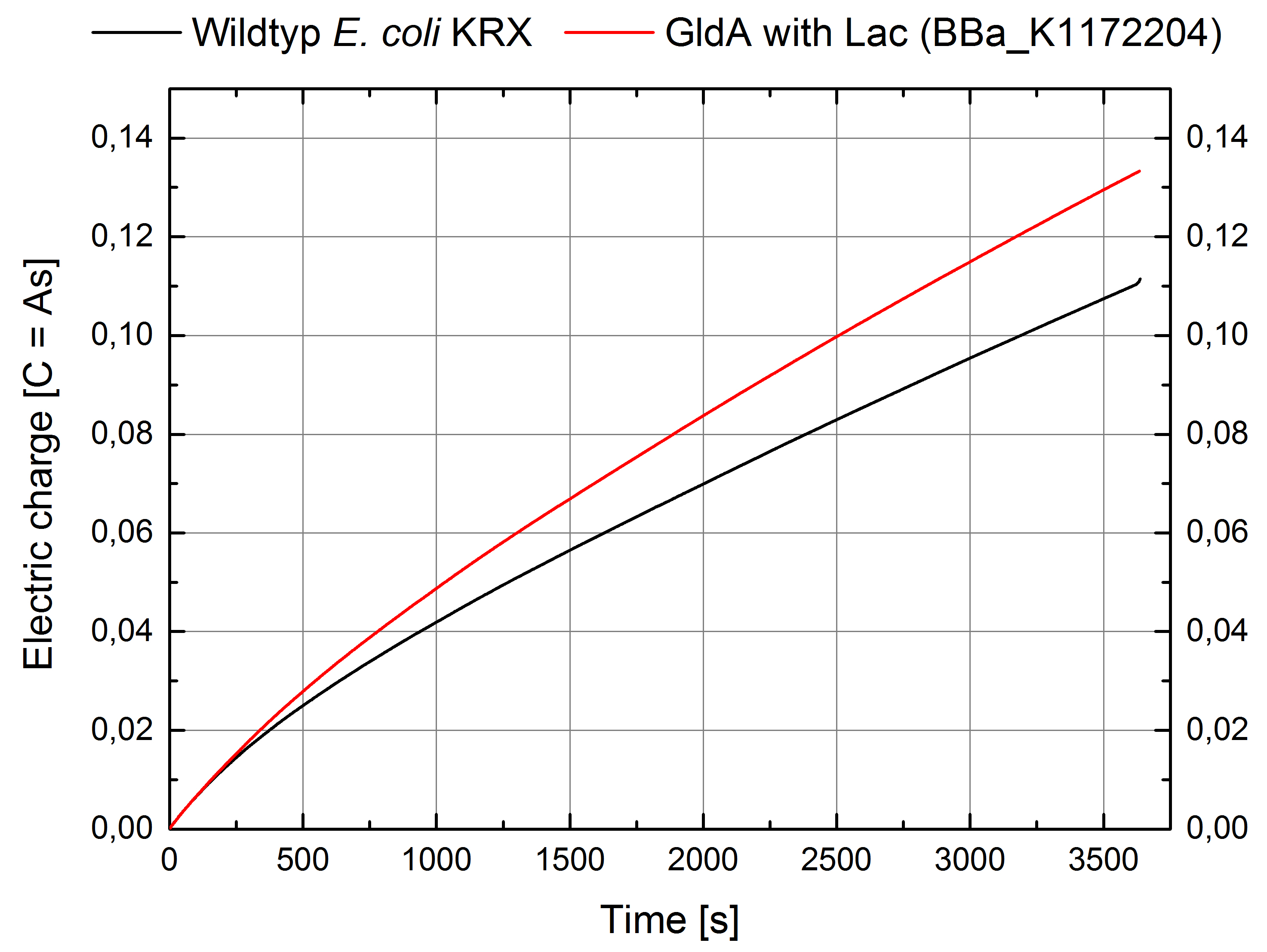
Figure 7: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with gldA (<bbpart>BBa_K1172204</bbpart>) in contrast to Escherichia coli KRX wild type. Electric charge curve from Escherichia coli KRX wild type and Escherichia coli KRX with gldA (<bbpart>BBa_K1172204</bbpart>) is shown over time. M9-medium was used with no supplementation of mediators.
- The extracellular electron transfer mediated by NADH is improved in the gldA strain resulting in an increased bioelectricity output.
Riboflavin
- We received the data from the MALDI-TOF and the subsequent results where positive: The overexpressed protein, had formed a band on a SDS-PAGE at about 15 kDa. We hoped it would turn out to be RibE, with a molecular weight of 16,7 kDa.
- The results show that it is indeed “riboflavin synthase beta subunit ribE” from Shewanella oneidensis MR-1. The mascot score was a slick 906. We want to thank Vera Ortseifen for the execution of our measurements.
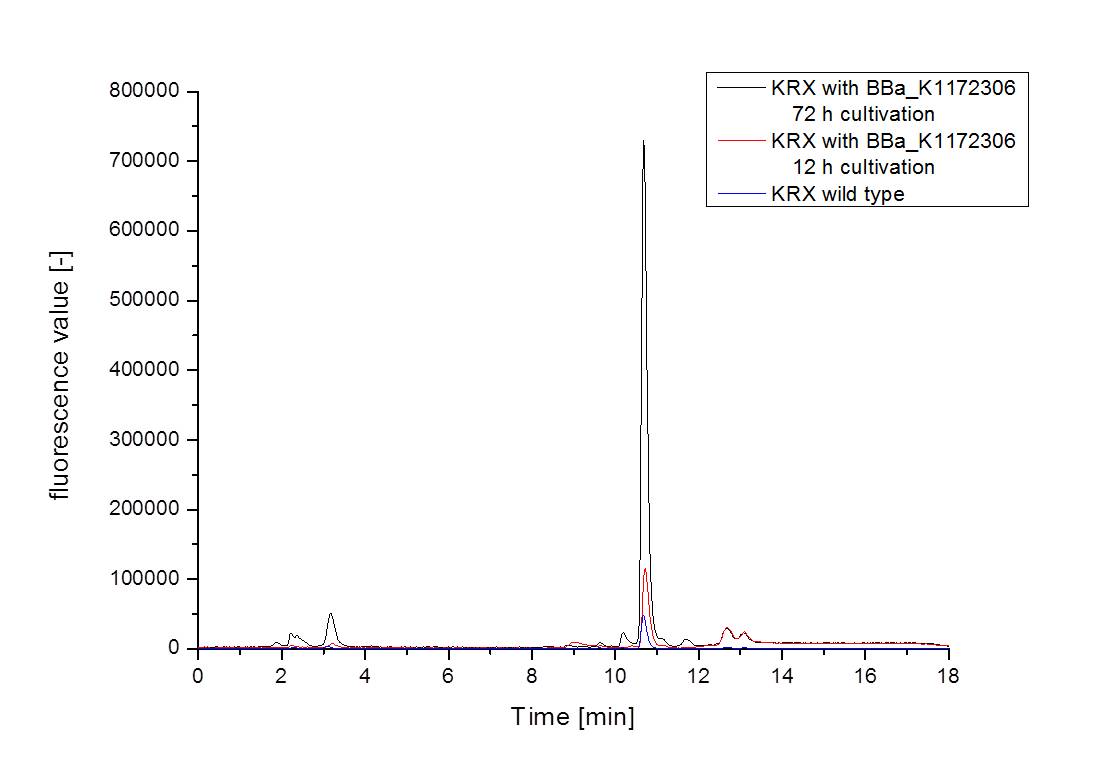
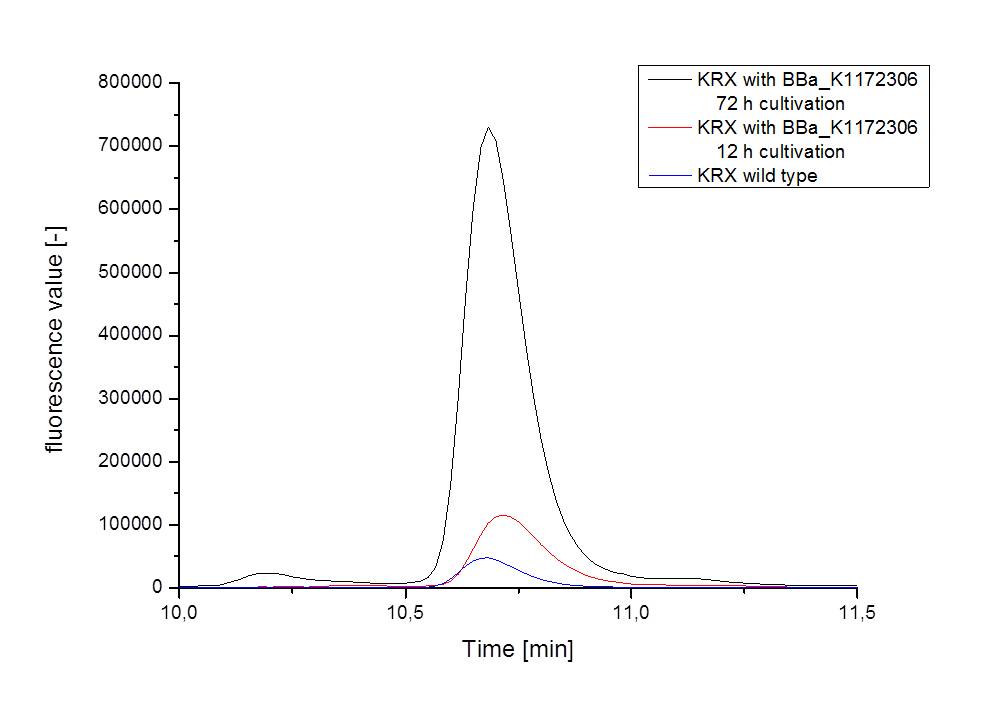
- For further quantitative analysis of riboflavin in culture supernatants we measured fluorescence with the [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader] (The week before we had already measured absorbance). Riboflavine absorbes light at 440 nm with a corresponding emission at 535 nm. We again checked riboflavin of known quantities to compute a calibration line. The samples consisted of KRX wild type bacteria (grown for 72 hours) and KRX that carried the rib-cluster with strong Anderson promoter / strong RBS (<bbpart>BBa_K1172306</bbpart>) (grown for 72 hours and for 12 hours respectively). Of course both cultures were nurtured in the good M9-D5.
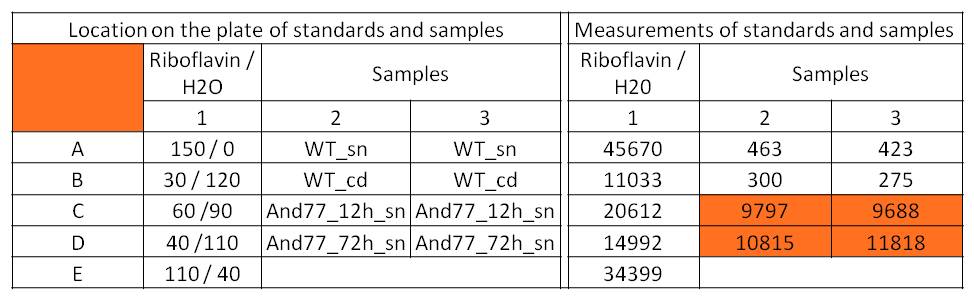
Table 5: Pipetting scheme and measurement results of riboflavin standards and cell samples for fluorescence measurement, emission at 535 nm. Measured in the [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]. WT = wild type, And77 = E. coli equipped with <bbpart>BBa_K1172306</bbpart>, sn = supernatant, cd = cell disruption.
- Y = 8*10^8 * X - 3193.6
- This allowed us to evaluate the amount of riboflavin in the supernatant and the cell disruption samples.
- Supernatant after 12 hours: 308.1 µg / L
- Supernatant after 72 hours: 3821.5 µg /L
- We asked the lab of Dr. Heino Büntemeyer if we could use their HPLC detector to verify riboflavin concentrations. We prepared some samples and overviewed the measurement. The HPLC-method itself was friendly carried out by Jana Heinrich. It yielded significant results and allows the quantification of riboflavins in our samples.
- Table 6 shows the produced amount of Riboflavin. After 72 hours of cultivation in M9-D5 the concentration of riboflavin in supernatant and cell disruption samples of E. coli KRX+<bbpart>BBa_K1172306</bbpart> was 60fold higher than in E. coli KRX wild type. Even after 12 hours, the riboflavin producing strain had generated ten times as much riboflavin as the wild type.
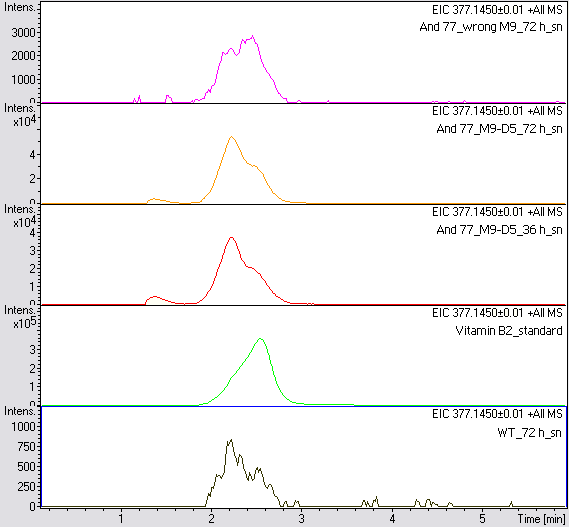
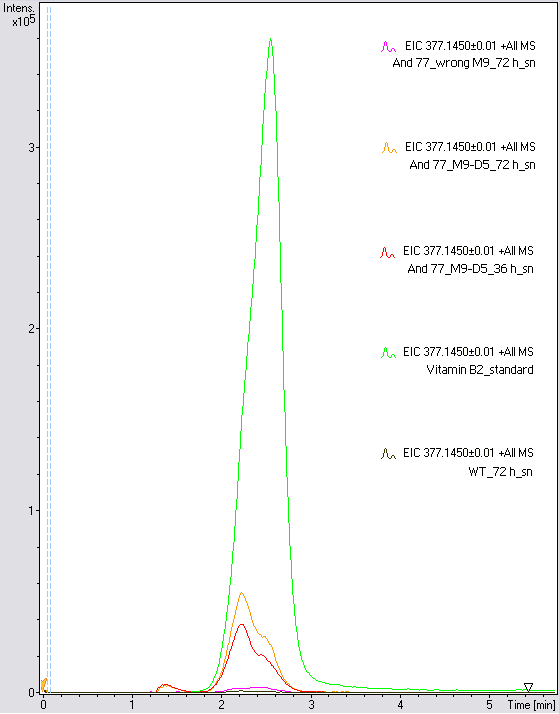
- The wrong M9 medium had been the reason for unsufficient riboflavin production. This fact rendered our first (LC/MS) measurement innocuous. We were able to organize another measurement of some of our samples with liquid chromatography-mass spectrometry (LC/MS). We prepared supernatant samples of a wild type KRX after 72 h, the <bbpart>BBa_K1172306</bbpart> strain after 36 h and 72 h in M9-D5 and after 72 h in a wrong M9. In the following we put the samples in the LC-MS-system. The measurement procedure was carried out by Lena Sinkel.
-
It is clear, that the <bbpart>BBa_K1172306</bbpart> carrying strains produce a much higher amount of riboflavin compared to the wild type KRX strain. The LC/MS results do not allow for a statement on how much more riboflavin our strain produces, because from this method we can´t know if the relation between the amount of vitamin B2 and the peak-area is linear. Nevertheless, it is obvious that riboflavin was overexpressed in a remarkable quantity.
It is although important to notice that the green curve in figures 18 and 19 represents the high concentrated riboflavin standard. A look at Figure 19 and the relative intensities given on the Y-axis, illustrates how much more efficient KRX+<bbpart>BBa_K1172306</bbpart> in M9-D5 produces riboflavin compared to KRX+<bbpart>BBa_K1172306</bbpart> in the wrong M9 and especially compared to wild type KRX.
-
Cytochromes
Biosafety
Porines
- Great results for Escherichia coli KRX with oprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type with Microbial Fuel Cell cultivation.
- According to our assumptions, the extracellular electron transfer mediated by electron shuttles is improved in the oprF strain resulting in an increased bioelectricity output. (Figure 1 and 2)
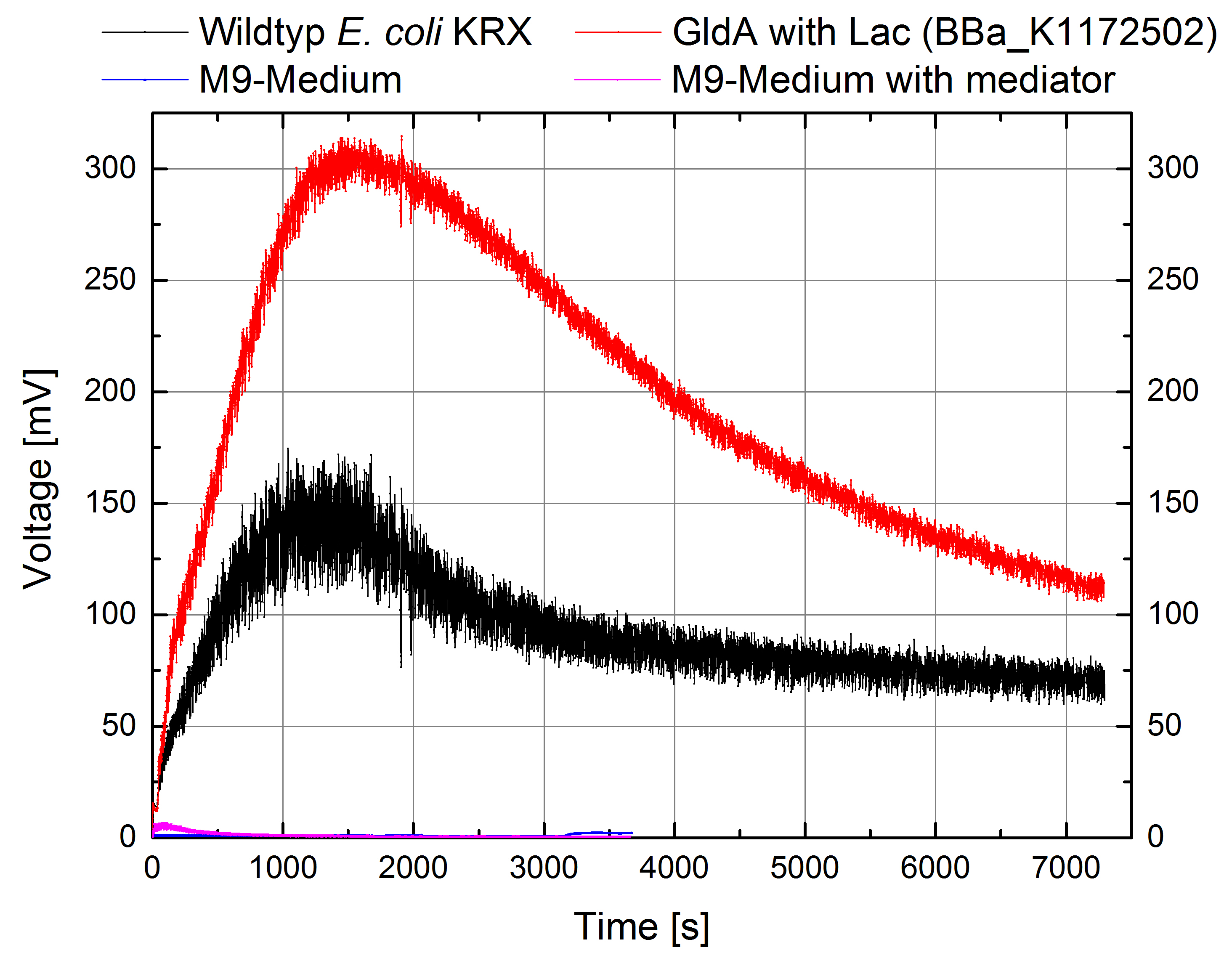
Figure 1: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with oprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type. Voltage curve from Escherichia coli KRX wild type, Escherichia coli KRX with oprF (<bbpart>BBa_K1172502</bbpart>), M9 medium with used mediator New Methylene Blue and M9 medium without mediator is shown over time.

Figure 2: Microbial Fuel Cell results from cultivation of Escherichia coli KRX with oprF (<bbpart>BBa_K1172502</bbpart>) in contrast to Escherichia coli KRX wild type. Electric charge curve from Escherichia coli KRX wild type and Escherichia coli KRX with oprF (<bbpart>BBa_K1172502</bbpart>). M9 medium was used with mediator New Methylene Blue.
- Escherichia coli KRX with oprF (<bbpart>BBa_K1172502</bbpart>) shows 100 % higher voltage and electric charge than E. coli wild type.
Week 23
Organization
MFC
Mediators
Cytochromes
Biosafety
Porines
Week 24
Organization
MFC
Mediators
Cytochromes
Biosafety
Porines
Week 25
Organization
MFC
Mediators
Cytochromes
Biosafety
Porines
 "
"