Team:Bielefeld-Germany/Biosafety/Biosafety System M
From 2013.igem.org
Biosafety System TetOR alive
Overview
The Biosafety-System TetOR alive <bbpart>BBa_K1172915</bbpart> is an improvement of the Biobrick <bbpart>BBa_K914014</bbpart> by changing the first promoter into the rhamnose promoter PRha, integration of the alanine racemase <bbpart>BBa_K1172901</bbpart> and usage of the repressor TetR to regulate the transcription of the Barnase behind the tetO promoter. Because this system is known for a tight repression and a fast activation, it is expected that bacteria containing this Biosafety-System are dead or alive...
Genetic Approach
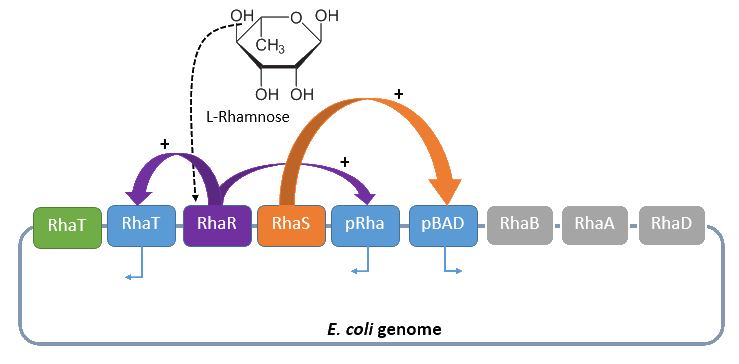
Rhamnose promoter PRha
The promoter PRha (<bbpart>BBa_K914003</bbpart>) naturally regulates the catabolism of the hexose L-rhamnose. The advantage of the operon for further usage is its solely positive regulation. The regulon consists of the promoter PRhaT, which regulates the expression of the protein RhaT for the uptake of L-rhamnose and the two operons rhaSR and rhaBAD.
The operon rhaSR regulates the genes rhaS and rhaR, whose translated proteins are responsible for the positive activation of the L-rhamnose catabolism, while the operon rhaBAD regulates the genes for the direct catabolism of L-rhamnose.
When L-rhamnose is present, it acts as an inducer by binding to the regulatory protein RhaR. RhaR regulates his own expression and the expression of the regulatory gene rhaS by inhibiting or, in the presence of L-rhamnose, activating the operon RhaSR. Normally the expression level is modest, but it can be enhanced by a higher level of intracellular cAMP, which increases in the absence of glucose. So, in the presence of L-rhamnose and a high concentration of intracellular cAMP, the activator protein RhaR is expressed on higher level, resulting in an activation of the promoter PRhaT for an efficient L-rhamnose uptake and an activation of the operon rhaBAD. The L-rhamnose is than broken down into dihydroxyacetone phosphate and lactate aldehyde by the enzymes of the operon rhaBAD (Wickstrum et al., 2005).
A brief schematic summary of the regulation is shown in Figure 2.
Dihydroxyacetone phosphate can be metabolized in the glycolysis pathway, while lactate aldehyde is oxidated to lactate under aerobic conditions and reduced to L-1,2,-propandiol under anaerobic conditions.
This degradation of L-rhamnose can be separated in three steps. In the first step the L-rhamnose is turned into L-rhamnulose by an isomerase (gene rhaA). The catabolism continues by the a kinase (gene rhaB), which phosphorylates the L-rhamnulose to L-rhamnulose-1-phosphate. This is finally hydrolyzed by Aldolase (gene rhaD) to dihydroxyacetone phosphate and lactate aldehyde (Baldoma et al., 1988).
Four our Safety-System the rhamnose promoter PRha is used to control the expression of the repressor AraC and the essential alanine racemase, because this promoter has an even lower basal transcription then the arabinose promoter PBAD. This is needed to tightly repress the expression of the alanine racemase (alr) and therefore take advantage of the double-kill switch. Although the rhamnose promoter PRha is characterized by a very low basal transcription it can not be used for the control of the toxic RNase Ba (Barnase), which ist needed for the alanine racemase (alr), the other part of the double-kill switch. In conclusion this promoter is the best choice for the first part of the Biosafety-System, as it is tightly repressed in the absence of L-Rhamnose, but activated in its presence.
Tetracyclin repressor/operator
The tetracycline repressor (TetR)/ operator (TetO) originally is used by E. coli to work against the antibiotic tetracycline but in many cases it is used for regulated expression for industrial processes. When there is no tetracycline available (continuous lines) the tetR gene is expressed and TetR binds as a dimer with high affinity the tetracycline operators, tetO1 and tetO2. When tetracycline diffuse through the cell membrane into E. coli (dotted lines) it aggregates with Mg2+ to a chelate complex (red triangle). The complex binds to TetR which switches its conformation and so it comes to a dissolution of the TetR and the TetO. Because of this the polymerase isn’t enhanced anymore and is able to express the tetA gene which is assembled into the cytoplasmic membrane where it works as an antiporter. It transports the complex out of the cell which makes the cell resistant against tetracycline (Saenger W. et al. ,2000).
In our system we use tetR (<bbpart>BBa_C0040</bbpart>) and tetO (<bbpart>BBa_R0040</bbpart>) but we don’t work with tetracycline so we haven’t got the conformation switch of TetR. Because of the fact that the promoter is induced in our microbial fuel cell (MFC) TetR is expressed and blocks TetO. If E. coli get out of the MFC the inducer isn’t available and tetR isn’t expressed any more. From now on the gene behind the operators is expressed which stands for a RNase, so E. coli dies (Saenger W. et al. ,2000).
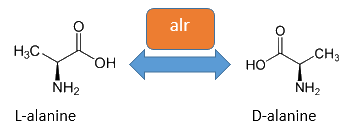
Alanine racemase Alr
The alanine racemase Alr (EC 5.1.1.1) from the gram-negative enteric bacteria Escherichia coli is a racemase, which catalyses the reversible conversion of L-alanine into the enantiomer D-alanine. For this reaction the cofactor pyridoxal-5'-phosphate (PLP) is typically needed. The constitutive expressed alanine racemase (alr) is naturally responsible for the accumulation of D-alanine, which is an essential component of the bacterial cell wall, because it is used for the crosslinkage of peptidoglykan (Walsh, 1989).
The usage of D-alanine instead of a typically L-amino acids prevents cleavage by peptidases. However a lack of D-alanine causes to a bacteriolytic characteristics. In the absence of D‑alanine dividing cells will lyse rapidly. This perception is used for our Biosafety-Strain, a D-alanine auxotrophic mutant (K-12 ∆alr ∆dadX). The Biosafety-Strain grows only with a plasmid containing the alanine racemase (<bbpart>BBa_K1172901</bbpart>) with a complementation of the D-alanine auxotrophy. Consequently the alanine racemase is essential for bacterial cell division. This approach guarantees a high plasmid stability, which is extremely important when the plasmid contains a toxic gene like the Barnase. In addition this construction provides the possibility for the implementation of a double kill-switch system. Because if the expression of the alanine racemase is repressed and there is no D-alanine-Supplementation in the media, cells will not grow.
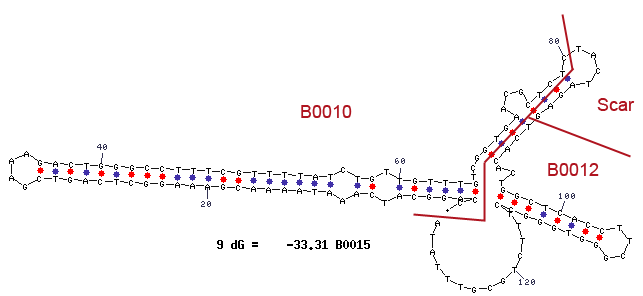
Terminator
Terminator are essential for the end of an operon. In prokaryotes exist rho depending and independing terminators. Rho independing terminators are characterized by an stem loop, which is caused by special sequence. In general the terminator region can be divided into four regions. Starting with a GC rich region, which performs the stem and followed by the loop region. The third region is made up from the opposite part of the stem, so that this region concerns also GC rich portion. After that the terminator ends by an poly uracil region, which destabilizes the binding of the RNA polymerase. The stem loop of the terminator causes a distinction of the DNA and the translated RNA, so that the binding of the RNA-polymerase is canceled and the transcription ends after the stem loop (Carafa et al., 1990).
For our safety system the terminator is necessary to avoid that the expression of the genes under the control of the Rhamnose promoter pRHA, like the Repressor araC and the alanine racemase (alr), are transcripted but not the genes of the arabinose promoter pBAD, which contains the toxic Barnase and would lead to cell death. We used the Biobrick <bbpart>BBa_B0015</bbpart>.
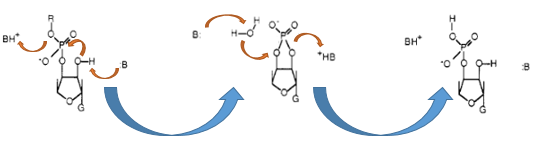
RNase Ba (Barnase)
The Barnase (EC 3.1.27) is a 12 kDa extracellular microbial ribonuclease, which is naturally found in the gram positive soil bacteria Bacillus amyloliquefaciens and consist a single chain of 110 amino acids. The Barnase (RNase Ba) catalysis the cleavage of single stranded RNA, where the hydrolysis of the dinucleotides has the highest affinity to the structure GpN. In the first step of the RNA-degradation a cyclic intermediate is formed by transesterification and afterwards this intermediate is hydrolysed yielding in a 3'-nucleotide (Mossakowska et al., 1989).
In Bacillus amyloliquefaciens the activity of Barnase (RNase Ba) is inhibited intracellular by the Inhibitor called barstar. Barstar consists only about 89 amino acids and binds with a high affinity to the toxic Barnase. This prevents the cleavage of the intracellular RNA in the host organism (Paddon et al., 1989). Therefore the Barnase acts naturally only outside the cell and is translocated under natural conditions. For the biosafety system we tried to modify this aspect by cloning only the sequence responsible for the cleavage of the RNA, but not the part of the native Barnase, which is essential for the extracellular transport.
As shown in the graphic below, the transcription of the DNA, which encodes the Barnase produces a 474 nt RNA. The translation of the RNA of this ribonuclease starts about 25 nucleotides downstream from the transcription start and can be divided into two parts. The first part (colored in orange) is translated into a signal peptide at the N-Terminus of the Barnase. This part is responsible for the extracellular translocation of the RNase Ba, while the peptide sequence for the active Barnase starts 142 nucleotides downstream from the transcription start (colored in red).
For the biosafety system we used only the coding sequence (<bbpart>BBa_K1172904</bbpart>) of the Barnase itself to prevent the extracellular translocation of the toxic gene product. This leads to a rapid cell death if the expression of the Barnase isn't repressed by the repressor of the biosafety system.
Biosafety system TetOR alive
Together with the Biosafety-Strain K-12 ∆alr ∆dadX the Biosafety-System TetOR alive takes advantage of this genes by combine them to a powerful device, which allows to control the bacterial cell division. The control of the bacterial growth is thereby active and passive possible. Active by conformation change of the tetracycline operator by adding tetracycline and passive by the induction of L-rhamnose. The passive control makes it possible to control the bacterial cell division in an defined environment, like the MFC by adding continously L-Rhamnose to the media. As shown in the figure below, this leads to an expression of the essential Alanine-Racemase (alr) and the tetracycline repressor, so that the expression of the RNase Ba is repressed.
When the bacteria exit the defined environment of the MFC or L-rhamnose is not added any more to the media, the expression of the alanine racemase (alr) and the tetracycline repressor decreases, so that the expression of the toxic RNase Ba (Barnase) is not inhibted so strong any more. The cleavage of the intracellular RNA by the Barnase and ideally also the lack of synthesized D-alanine, caused by the repressed alanine racemase inhibits the cell division and makes sure that the bacteria can only grow in the defined area.

Results
Characterization of the tetracycline tetO operator
First of all the bacterial growth under the pressure of the unrepressed tetracycline operator TetO was investigated on different carbon source. Therefore the cultivation on M9 minimal media with glycerin or glucose was characterized. To identify the transcription rate of the unexpressed tetracycline operator TetO the expression of the green fluorescence protein GFP BBa_E0040 behind the tetracycline operator TetO was used.
As shown in figure 10 below, the bacteria adapted better on glucose then on glycerol. As glucose is the more powerful energy source, because it posses more carbon atoms than glycerol these result was expected before. So more interesting are the fluorescence measurement shown in the figure 11. As it can be seen also the fluorescence depends on the carbon source, but not as strong as it can be seen by the arabinose promoter pBAD. The Biosafety-Strain K-12 ∆alr ∆dadX shows a little higher expression of GFP with glycerol than with glucose. This difference between the two curves can be seen as insignificant.

It can be seen that the expression of GFP between the two cultivations doesn’t have that difference like the systems Aractive or the lac of growth have. As the production rate by using glucose as carbon source is nearly the same like the cultivation with glycerol. As the specific production rate was calculated between every single measurement point the curve is not smoothed and so the fluctuations have to be ignored, as they do not stand for are real fluctuations in the transcription in the expression of GFP. They are caused by the growth curve and the fluorescence curve. And as they are not ideal there exists the fluctuations. But this graph shows that there is an insignificant difference between the two carbon sources.
Characterization of the Biosafety-System TetOR alive
The Biosafety-System TetOR alive was characterized on M9 minimal medium using glycerol as carbon source. As for the characterization of the pure tetracycline promoter above, the bacterial growth and the fluorescence of GFP <bbpart>BBa_E0040</bbpart> was measured. Therefore, the wild type and the Biosafety-Strain E. coli K-12 ∆alr ∆dadX containing the Biosafety-Plasmid <bbpart>BBa_K1172912</bbpart> were cultivated once with the induction of 1% L-rhamnose and once only on glycerol.
It becomes obvious (Figure 13) that the bacteria, induced with 1 % L-rhamnose (red and black curve) grow significantly slower than on pure glycerol (yellow and blue curve). This is attributed to the high metabolic burden encountered by the induced bacteria. The expression of the repressor AraC and the alanine racemase (Alr) simultaneously causes a high metabolic stress on the cells, so that they grow slower than the uninduced cells, who expresses only GFP. Additionally the tetracycline promoter is tightly regulated, so that the expression even with a small amount of the repressor AraC is not that high and therefore not as stressful as the induced expression of the AraC repressor and the alanine racemase (Alr).
Comparing the bacterial growth with the fluorescence in figure 14 it can be seen that the fluorescence of the Biosafety-Strain can not be evaluate because of the long duration of the lac-phase.
From the figure above it can not be seen if the expression of the repressor TetR does effect the transcription of GFP or not. The slower growth of the bacteria is a first indication that the repressor tetR and the alanine racemase are highly expressed. As the specific production rate was calculated between every single measurement point the curve is not smoothed and so the fluctuations have to be ignored, as they do not stand for are real fluctuations in the transcription in the expression of GFP. They are caused by the growth curve and the fluorescence curve. And this measured curves are not ideal the calculation of the specific production rate causes the fluctuations. But it can be seen that the production of GFP differs and tends to be lower, when the bacteria are induced with 1% L-Rhamnose. So the Biosafety-System tetOR alive works.
Conclusions
References
- Saenger W. et al. (2000):Der Tetracyclin-Repressor – das Musterbeispiel für einen biologischen Schalter, In:[http://onlinelibrary.wiley.com/doi/10.1002/1521-3757%2820000616%29112:12%3C2122::AID-ANGE2122%3E3.0.CO;2-8/abstract Angewandte Chemie Volume 112, Issue 12, pages 2122–2133]
- Mossakowska, Danuta E. et al. (1989): Kinetic Characterization of the Recombinant Ribonuclease from Bacillus amyloliquefaciens (Barnase) and Investigation of Key Residues in Catalysis by Site-Directed Mutagenesis [http://pubs.acs.org/doi/pdf/10.1021/bi00435a033 Biochemistry 28: 3843 - 3850].
- Paddon, C. J. Vasantha, N. and Hartley, R. W. (1989): Translation and Processing of Bacillus amyloliquefaciens Extracellular Rnase [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC209718/pdf/jbacter00168-0575.pdf|Journal of Bacteriology 171: 1185 - 1187].
- Voss, Carsten Lindau, Dennis and Flaschel, Erwin (2006): Production of Recombinant RNase Ba and Its Application in Downstream Processing of Plasmid DNA for Pharmaceutical Use [http://onlinelibrary.wiley.com/doi/10.1021/bp050417e/pdf|Biotechnology Progress 22: 737 - 744].
- Orth P. et al. (2000): Structual basis of gene regulation by the tetracycline inducible Tet repressor-operator system. In: [http://life.nthu.edu.tw/~b871641/tetrepressor.pdf nature structural biology, volume 7 number 3].
 "
"