Team:Bielefeld-Germany/Datapage
From 2013.igem.org
Data Page
Overview
This page gives a basic overview about our our project Ecolectricity - currently available. A detailed description of our subprojects can be found in our project part of the Wiki.
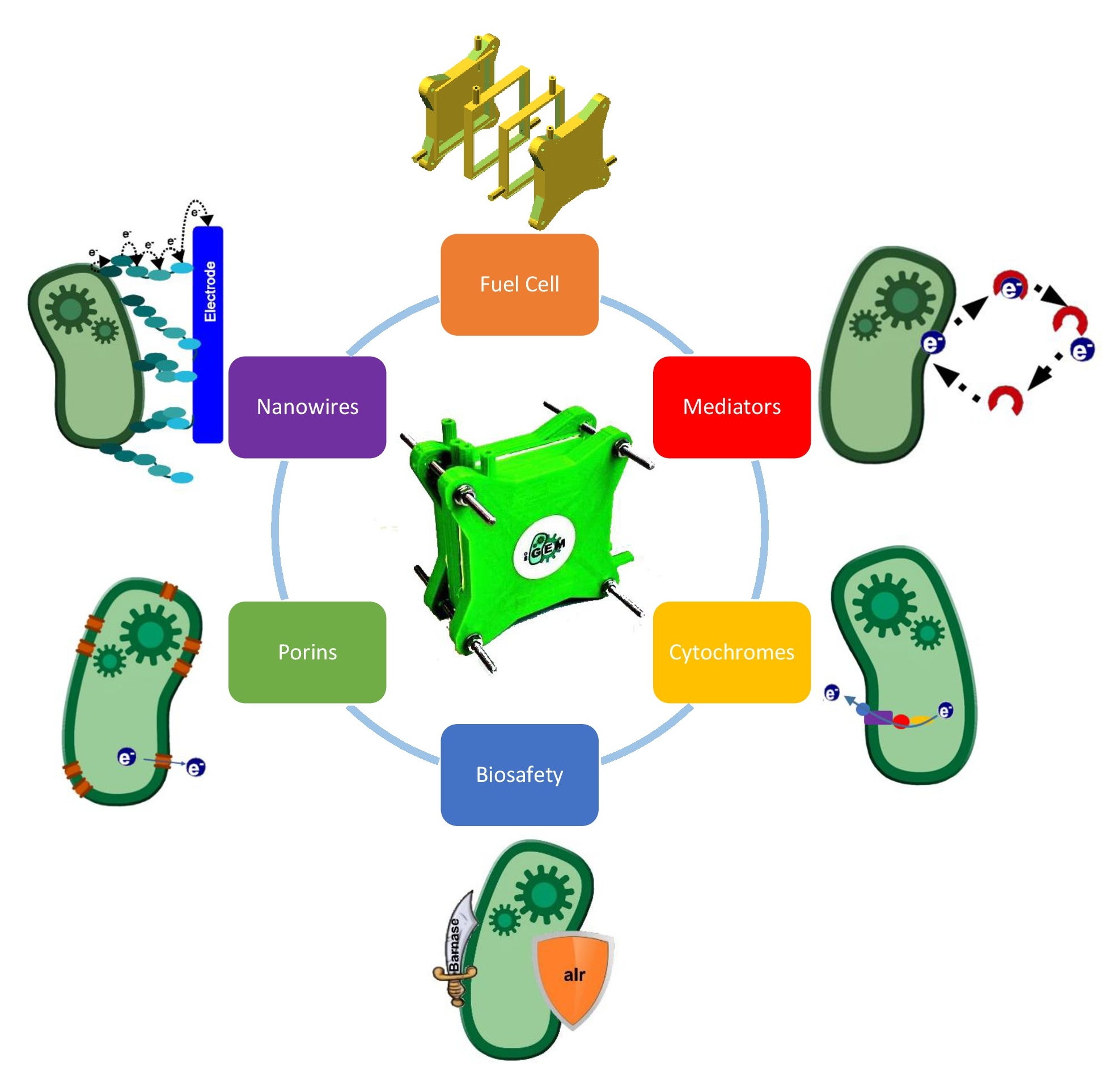
Project description: The goal of our project is to generate electrical energy with a genetically modified Escherichia coli in a self-constructed Fuel Cell. Besides the design, construction and technical optimization of the fuel cell, we investigate different genetic approaches. Specific electron transfer proteins have been compiled from a variety of organisms, in order to gain an Escherichia coli Fuel Cell platform which turns E. coli to an electro active organism. The main challenge is to ensure an efficient electron transfer from the bacteria to the electrode. Therefore we facilitate and improve electron donation by producing electron-shuttles, so called endogenous mediators, as well as permeabilizing the cell surface by integrating large membrane porins and providing a direct electron pathway by conductive transmembrane protein structures. All these electron transport elements increase electron transfer and bioelectricity generation.
Data for our favorite new parts
- <bbpart>BBa_K1172501</bbpart> - OprF – Outer membrane porin from Pseudomonas fluorescens: This outer membrane porin enhances membrane permeability and electron shuttle-mediated extracellular electron transfer for higher bioelectricity output.
- <bbpart>BBa_K1172901</bbpart> - Alanine racemase from E. coli: Catalyzes the reversible isomerization from L-alanine into the enatiomer D-alanine. In an D-alanine auxotrophic strain it can be used as a antibiotic free selection marker.
- <bbpart>BBa_K1172303</bbpart> - Riboflavin synthesis gene cluster from Shewanella oneidensis: Used to produce vitamin B2 which can act as an endogenous mediator.
- <bbpart>BBa_K1172909</bbpart> - Biosafety System araCtive: Biosafety System taking advantage of the alanine racemase and the tight repression of the arabinose promoter ParaBAD by the AraC protein.
We have also characterized the following parts (Coding)
- <bbpart>BBa_K1172201</bbpart> - GldA - Glycerol dehydrogenase from Escherichia coli: Glycerol dehydrogenase can be used for endogenous mediator production of NADH. Higher amount of electron shuttles improve transfer of electrons from bacteria to the anode and makes the MFC more efficient.
- <bbpart>BBa_K1172301</bbpart> - NorM: Na+ antiporter from Shewanella oneidensis: MATE_NorM_like; Subfamily of the multidrug and toxic compound extrusion (MATE)-like proteins similar to Vibrio cholerae NorM.
- <bbpart>BBa_K1172302</bbpart> - RibC: 6,7-dimethyl-8-ribityllumazine synthase alpha subunit RibC from Shewanella oneidensis: Gene has homologous properties compared to ribE, which is part of the riboflavin synthesis gene cluster.
- <bbpart>BBa_K1172401</bbpart> - MtrCAB – Cytochromes from Shewanella oneidensis: Mtr proteins for direct electron transfer to the outer cell membrane via c-type cytochromes.
- <bbpart>BBa_K1172904</bbpart> - Rnase Ba (Barnase) from Bacillus amyloliquefaciens: Genetically modified variant that cleaves the intracellular RNA of the host organism.
- <bbpart>BBa_K1172916</bbpart> - Double Plac promoter: Improved repression of the lac promoter by an additional LacI-binding site, 1.5 helix turns downstream of the natural binding site.
We have also characterized the following parts (Devices)
Endogenous mediator prodcution by glycerol dehydrogenase
- <bbpart>BBa_K1172203</bbpart> - gldA with T7 promoter and strong RBS: This glycerol dehydrogenase device is used to characterize the endogenous mediator production of NADH and seems to be the best part for efficient NADH production.
- <bbpart>BBa_K1172204</bbpart> - gldA with IPTG inducible lac promoter and strong RBS: This glycerol dehydrogenase device is used to characterize the endogenous mediator production of NADH.
- <bbpart>BBa_K1172205</bbpart> - gldA with medium Anderson promoter and medium RBS: This glycerol dehydrogenase device is used to characterize the endogenous mediator production of NADH.
Porins
- <bbpart>BBa_K1172502</bbpart> - oprF with T7 promoter and strong RBS: This device is used to characterize the outer membrane porin expression and its effect on the cell surface permeability. This part is the most efficient OprF expression device leading to the highest bioelectricity output.
- <bbpart>BBa_K1172503</bbpart> - oprF with IPTG inducible lacI promoter and strong RBS: This device is used to characterize the outer membrane porin expression and its effect on the cell surface permeability.
- <bbpart>BBa_K1172504</bbpart> - oprF with medium Anderson promoter and weak RBS: This device is used to characterize the outer membrane porin expression and its effect on the cell surface permeability.
- <bbpart>BBa_K1172505</bbpart> - oprF with medium Anderson promoter and medium RBS: This device is used to characterize the outer membrane porin expression and its effect on the cell surface permeability.
- <bbpart>BBa_K1172507</bbpart> - oprF with strong Anderson promoter and strong RBS: This device is used to characterize the outer membrane porin expression and its effect on the cell surface permeability.
Endogenous mediator Riboflavin
- <bbpart>BBa_K1172304</bbpart> - Riboflavin synthesis gene cluster from Shewanella oneidensis with T7 promoter and strong RBS: Riboflavin synthesis gene cluster, induced by rhamnose in E. coli KRX.
- <bbpart>BBa_K1172305</bbpart> - Riboflavin synthesis gene cluster from Shewanella oneidensis with medium Anderson promoter and medium RBS: Riboflavin synthesis gene cluster under control of medium anderson promoter.
- <bbpart>BBa_K1172306</bbpart> - Riboflavin synthesis gene cluster from Shewanella oneidensis with strong Anderson promoter and strong RBS: This device was used for extensive quantitative and qualitative proof of riboflavin overproduction.
Cytochromes
- <bbpart>BBa_K1172403</bbpart> - mtrCAB with medium promoter and medium RBS: This device is coding for cytochromes MtrA, MtrB and MtrC, leading to direct electron transfer through the outer membrane.
- <bbpart>BBa_K1172404</bbpart> - mtrCAB with strong promoter and strong RBS: This device is coding for cytochromes MtrA, MtrB and MtrC, leading to direct electron transfer through the outer membrane.
- <bbpart>BBa_K1172405</bbpart> - mtrCAB with T7 promoter and strong RBS: This device is coding for cytochromes MtrA, MtrB and MtrC, leading to direct electron transfer through the outer membrane.
Biosafety-System
- <bbpart>BBa_K1172902</bbpart> - Alanine racemase (alr) under the control of the Ptac promoter: Testing part for the complementation of the alanine racemase (alr) in the Biosafety-Strain E. coli K-12 ∆alr ∆dadX to ensure that the alanine racemase could be used as a selection marker instead of antibiotics.
- <bbpart>BBa_K1172903</bbpart> - Alanine racemase (alr) with double terminator: Cloning intermediate of the construction of the Biosafety-Strains. This might part is be useful for other teams, who want to work with the alanine racemase (alr).
- <bbpart>BBa_K1172905</bbpart> - Part 1 of the Biosafety- System araCtive: Front part of the Biosafety-System araCtive containing: Prha - araC - alr - ter.
- <bbpart>BBa_K1172906</bbpart> - Part 2 of the Biosafety-System araCtive (ParaBAD Barnase): Second part of the Biosafety-System araCtive containing ParaBAD RNase Ba.
- <bbpart>BBa_K1172908</bbpart> - Part 1 of the Biosafety-System Lac of Growth: Front part of the Biosafety-System Lac of Growth containing: Prha - lacI - alr - ter.
- <bbpart>BBa_K1172911</bbpart> - Biosafety-System Lac of Growth (LacI): Biosafety-System Lac of Growth using LacI as repressor and the lactose promoter.
- <bbpart>BBa_K1172912</bbpart> - Part 1 of the Biosafety-System TetOR alive: Front part of the Biosafety-System TetOR alive containing: Prha - tetR - alr - ter.
- <bbpart>BBa_K1172914</bbpart> - Part 2 of the Biosafety-System TetOR alive with gfp (TetO gfp): Second part of the Biosafety-System TetOR alive containing TetO and gfp (<bbpart>BBa_E0040</bbpart>).
- <bbpart>BBa_K1172915</bbpart> - Biosafety-System TetOR alive (TetR): Biosafety-System TetOR alive using TetR as repressor and the tetO promoter/operator region.
- <bbpart>BBa_K1172917</bbpart> - Double Plac promoter with gfp : Improved repression of the lac promoter by an additional LacI-binding site, 1.5 helix turns downstream of the natural binding site.
Part combinations
- <bbpart>BBa_K1172566</bbpart> - OprF and GldA (Coding): Combination of the outer membrane porin OprF (<bbpart>BBa_K1172501</bbpart>) and the glycerol dehydrogenase GldA (<bbpart>BBa_K1172201</bbpart>).
- <bbpart>BBa_K1172577</bbpart> - OprF and GldA (Coding): Combination of the outer membrane porin OprF (<bbpart>BBa_K1172502</bbpart>) and the glycerol dehydrogenase GldA (<bbpart>BBa_K1172201</bbpart>) under control of T7 promoter.
- <bbpart>BBa_K1172588</bbpart> - OprF and Riboflavin synthesis gene cluster (Coding): Combination of the outer membrane porin OprF (<bbpart>BBa_K1172501</bbpart>) and Riboflavin synthesis gene cluster (BBa_K1172303).
- <bbpart>BBa_K1172599</bbpart> - OprF and Riboflavin synthesis gene cluster (Coding): Combination of the outer membrane porin OprF (<bbpart>BBa_K1172502</bbpart>) and the Riboflavin synthesis gene cluster (BBa_K1172303) under control of T7 promoter.
 "
"


