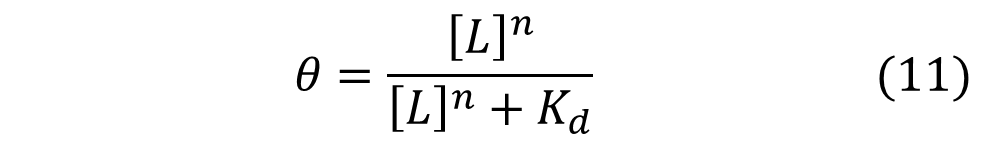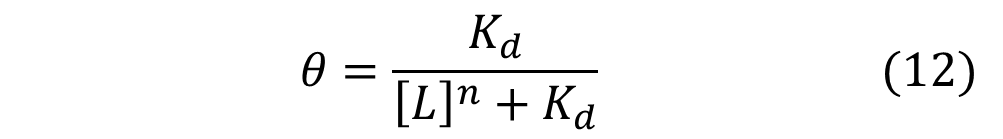Team:Duke/Modeling/Cooperativity
From 2013.igem.org
Contents |
Mathematical Modeling of Bistable Toggle Switch
Cooperativity
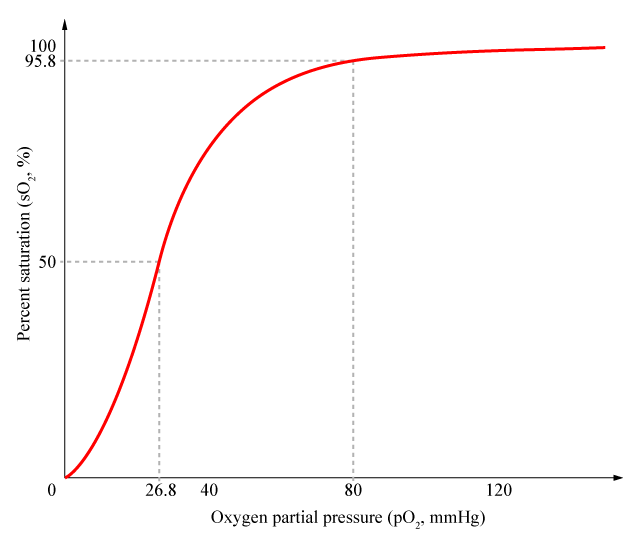
Cooperativity is a common phenomenon in biological systems involving multiple ligands binding to enzymes or receptors. The change in affinity of a binding site for a ligand upon a ligand-binding leads to either positive cooperativity in which affinity is increased and subsequent binding become more likely, or negative cooperativity in which affinity is decreased to hinder future binding. Cooperativity is critical for ideal gene circuits’ function because high cooperativity can lead to sudden change in states (i.e. fast switch between “off” state and “on” state). One of the most famous examples of cooperativity in biological systems is the binding of oxygen to hemoglobin, where its affinity for oxygen increases after each additional oxygen-binding. As a result, cooperativity results in non-linear and sigmoidal shaped curves.
It has been shown by both mathematical derivation and experimental data that proteins which form multimers have cooperativity (Phillips et al, 2012). For example, a transcriptional repressor of the lac operon, lacI proteins form tetramers and show cooperative repression of transcription. Similarly, tetracycline repressor (tetR) found in E.coli form dimers, and as a result show cooperativity. The nonlinearity of cooperativity can be explained by using rate equations for multimerization.
For a repressor that must multimerize to become active, the concentration of the multimer species effectively drives the reaction. As equation (3) shows, the concentration of dimer is proportional to the square of the concentration of monomer when in equilibrium. Similarly, equation (6) shows that the concentration of tetramer is proportional to the 4th power of the concentration of monomer. As a result, a linear increase in monomer concentration leads to nonlinear increase of the concentration of active multimerized-repressors, and thus shows cooperativity.
In addition to multimerization, combinatorial promoter binding has been shown to generate cooperativity (Murphy et al, 2007).
Assuming that all binding site with one or more repressors bound can repress RNA polymerase from binding, results similar to that of multimerization can be derived. Shown above is an example derviation for a promoter with three repressor binding sites. As shown in the equations, the level of effective species (promoter with one, two or three repressors bound) depend on the cube of level of repressors.
Murphy has shown that having multiple operator sites for both inducers and repressors produced cooperative response and it is this design that we chose to use in our project (with multiple binding sites for TALEs and sgRNA-dCas9). Cooperativity of repressors with multiple binding sites will be further explored in our mathematical models.
Background Knowledge
Cooperativity
Cooperativity is a common phenomenon in biological systems involving multiple ligands binding to enzymes or receptors. The change in affinity of a binding site for a ligand upon a ligand-binding leads to either positive cooperativity in which affinity is increased and subsequent binding become more likely, or negative cooperativity in which affinity is decreased to hinder future binding. One of the most famous examples of cooperative binding is the binding of oxygen to hemoglobin, the oxygen-transporting protein in red blood cells. When an oxygen molecule bind to hemoglobin, its affinity for oxygen increases greatly, and when three oxygen molecules bind (3-oxy-hemoglobin), its affinity for the fourth one is nearly three-hundred times higher than deoxy-hemoglobin’s affinity for oxygen. This cooperative property leads to the sigmoidal shaped curve shown below.
It has been shown by both mathematical derivation and experimental data that proteins which form multimers have cooperativity (Phillips et al, 2012). For example, a transcriptional repressor of the lac operon, lacI proteins form tetramers and show cooperative repression of transcription. Similarly, tetracycline repressor (tetR) found in E.coli form dimers, and as a result show cooperativity.
In addition to multimerization, combinatorial promoter binding has been shown to generate cooperativity (Murphy et al, 2007). Having multiple operator sites for both inducers and repressors produced cooperative response and it is this design that we chose to use in our project (with multiple binding sites for iTALs and CRISPR-Cas9). Cooperativity of repressors with multiple binding sites will be further explored in our mathematical models.
Hill Equation
Named after English physiologist Archibald Vivian Hill who studied the sigmoidal binding curve of hemoglobin, the Hill Equation provides a way to quantify cooperativity. The equation describes the fraction of proteins or enzymes that are saturated by ligands as a function of the concentration of ligands.
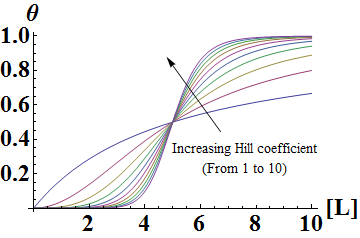
In the Hill Equation shown above, theta represents the fraction of binding sites occupied by ligands, [L] is the ligand concentration, n is the Hill coefficient, and Kd is the dissociation constant of the ligand. The value of the Hill coefficient describes the cooperativity of ligand binding: n>1 for positive cooperativity, n<1 for negative cooperativity and n=0 for no cooperativity. The above equation can also be applied to cooperative induction or activation of transcription, where theta represents the level of mRNA produced and [L] represents the level of inducer present. More cooperative the activation is, the steeper the slope will be, and the expression of the gene becomes more switch-like with distinct on-and-off states without intermediate states. Shown below is the graph the of Hill equation with various Hill coefficients. As one can see, increasing the Hill coefficient makes the change (induction) more abrupt and switch-like.
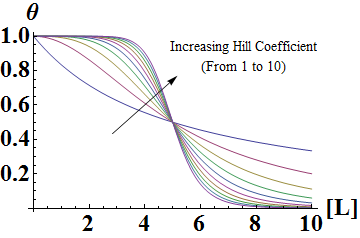
Similarly, mathematical derivation of a system with repressors that cooperatively repress a gene expression can be modeled by the following equation shown below. Again, Theta represents the level of transcribed mRNA, and [L] represents the level of repressors present. The graph of the above equation is shown as well. Similar to the previous graph, the level of transcription switches more abruptly with higher cooperativity (higher Hill coefficient). Therefore, as shown in both cases, high cooperativity lead to switch-like behavior and is favorable when designing a bistable toggle switch.
References
- Phillips R, Kondev J, Theriot J, Garcia H: Physical Biology of the Cell. Taylor & Francis; 2012.
- Murphy K.F., Balazsi G., Collins J.J.: Combinatorial promoter design for engineering noisy gene expression. Proc. Natl. Acad. Sci. USA. 2007, 104:pp. 12726–12731
Modeling Pages
- Cooperativity and Hill Equation (You are here)
- Thermodynamic Model: Introduction
- Thermodynamic Model: Application
- Kinetic Model
Supplementary Materials
 "
"